Volume 7, Number 1—February 2001
Synopsis
Geographic Subdivision of the Range of the Malaria Parasite, Plasmodium vivax
Figure 4
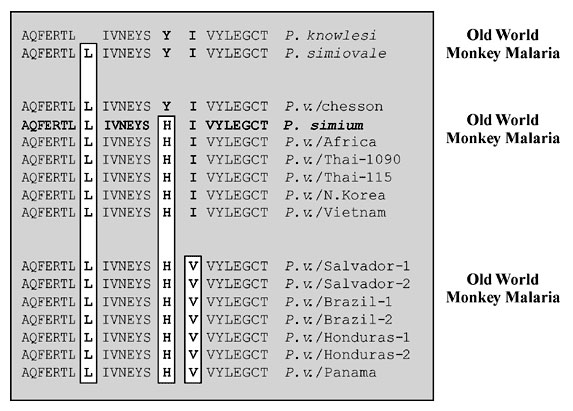
Figure 4. Polymorphism in the ORF 470 region of the 35-kb plastid-like DNA was determined by DNA sequence analysis after amplification of DNA from each isolate with oligonucleotide primers #1274 (5'GTAAAATTATATAAACCACC3') and #1273 (5'GCACAATTTGAACGTAC3') (11).
References
- Bruce-Chwatt LJ. Essential malariology. New York: John Wiley and Sons; 1985.
- Mayr E. Animal species and evolution. Cambridge: Belknap Press of Harvard University Press; 1963.
- Killick-Kendrick R. In: Peters W, editor. Rodent malaria. London: Academic Press; 1978.
- Collins WE, Skinner JC, Pappaioanou M, Ma NS, Broderson JR, Sutton BB, Infection of Aotus vociferans (karyotype V) monkeys with different strains of Plasmodium vivax. J Parasitol. 1987;73:536–40. DOIPubMedGoogle Scholar
- Collins WE, Warren M, Huong AY, Skinner JC, Sutton BB, Stanfill PS. Studies of comparative infectivity of fifteen strains of Plasmodium vivax to laboratory-reared anopheline mosquitoes, with special reference to Anopheles culicifacies. J Parasitol. 1986;72:521–4. DOIPubMedGoogle Scholar
- Collins WE, McClure H, Strobert E, Skinner JC, Richardson BB, Roberts, et al. Experimental infection of Anopheles gambiae s.s., Anopheles freeborni, and Anopheles stephensi with Plasmodium malariae and Plasmodium brasilianum. J Am Mosq Control Assoc. 1993;9:68–71.PubMedGoogle Scholar
- Li J, Wirtz RA, McCutchan TF. Analysis of malaria parasite RNA from decade-old giemsa-stained blood smears and dried mosquitoes. Am J Trop Med Hyg. 1997;57:727–31.PubMedGoogle Scholar
- Li J, Wirtz RA, McConkey GA, Sattabongkot J, McCutchan TF. Transition of Plasmodium vivax ribosome types corresponds to sporozoite differentiation in the mosquito. Mol Biochem Parasitol. 1994;65:283–9. DOIPubMedGoogle Scholar
- Wirtz RA, Burkot TR, Andre RG, Rosenberg R, Collins WE, Roberts DR. Identification of Plasmodium vivax sporozoites in mosquitoes using an enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1985;34:1048–54.PubMedGoogle Scholar
- Li J, Wirtz RA, McConkey GA, Sattabongkot J, Waters AP, Rogers MJ, Plasmodium: genus-conserved primers for species identification and quantitation. Exp Parasitol. 1995;81:182–90. DOIPubMedGoogle Scholar
- Wilson RJM, Denny PW, Preiser PR, Rangachari K, Roberts K, Roy A, Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J Mol Biol. 1996;261:155–72. DOIPubMedGoogle Scholar
- Waters AP, McCutchan TF. Partial sequence of the asexually expressed SU rRNA gene of Plasmodium vivax [published erratum appears in Nucleic Acids Res 1989 May 11;17:3630-1]. Nucleic Acids Res. 1989;17:2135. DOIPubMedGoogle Scholar
- Escalante A, Barrio E, Ayala FJ. Evolutionary origin of human and primate malarias: evidence from the circumsporozoite protein gene. Mol Biol Evol. 1995;12:616–26.PubMedGoogle Scholar
- Vaidya AB, Morrisey J, Plowe CV, Kaslow DC, Wellems TE. Unidirectional dominance of cytoplasmic inheritance in two genetic crosses of Plasmodium falciparum. Mol Cell Biol. 1993;13:7349–57.PubMedGoogle Scholar
- Gupta S, Ferguson N, Anderson R. Chaos, persistence and evolution of strain structure in antigenically diverse infectious agents. Science. 1998;280:912–5. DOIPubMedGoogle Scholar
- Lal AA, de la Cruz VF, Collins WE, Campbell GH, Procell PM, McCutchan TF. Circumsporozoite protein gene from Plasmodium brasilianum. Animal reservoirs for human malaria parasites? J Biol Chem. 1988;263:5495–8.PubMedGoogle Scholar
- Escalante AA, Freeland DE, Collins WE, Lal AA. The evolution of primate malaria parasites based on the gene encoding cytochrome-b from the linear mitochondrial genome. Proc Natl Acad Sci U S A. 1998;95:8124–9. DOIPubMedGoogle Scholar
- Kain KC, Brown AE, Webster HK, Wirtz A, Keystone JS, Rodriguez MH, Circumsporozoite genotyping of global isolates of Plasmodium vivax from dried blood specimens. J Clin Microbiol. 1992;30:1863–6.PubMedGoogle Scholar
- Simpson GC. Tempo and mode in evolution. New York: Columbia University Press; 1944.
- Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias. Bethesda: Dept of Health, Education and Welfare (US); 1971.
- Gonzalez-Ceron L, Rodriquez MH, Nettel JC, Villarreal C, Kain KC, Hernandez JE. Differential susceptibilities of Anopheles albimanus and An. pseudopunctipennis to infections with coindigenous Plasmodium vivax variants VK210 and VK247 in southern Mexico. Infect Immun. 1999;67:410–2.PubMedGoogle Scholar
¹The biologic diversity inherent in P. vivax already justifies the use of a trinomial system for naming its members that includes the designation of subspecies, a taxonomic character given formal recognition in the International Rules of Zoological Nomenclature. A subspecies is a population or group of populations inhabiting a geographic subdivision of the range of a species and differing from other populations by diagnostic morphologic characteristics.
²The designation of separate species does not require that the two organisms cannot mate and produce viable progeny, only that this does not happen with frequency in natural situations.