Volume 10, Number 12—December 2004
Dispatch
Naturally Acquired Plasmodium knowlesi Malaria in Human, Thailand
Cite This Article
Citation for Media
Abstract
We describe a case of naturally acquired infection with Plasmodium knowlesi in Thailand. Diagnosis was confirmed by the small subunit ribosomal RNA and the mitochondrial cytochrome b sequences. The occurrence of simian malaria in human has signified the roles of wild primate populations in disease transmission in some malaria-endemic areas.
A number of emerging pathogens have been known to cross-transmit between humans and nonhuman hosts. Wild primate populations have the potential to serve as origins and reservoirs of certain human pathogens, ranging from virus to helminths (1). More than 26 species of Plasmodium circulate among primate populations (2). Several of the simian malaria species are closely related to the human ones, and some of these, e.g. Plasmodium simium, P. brasilianum, P. cynomolgi, P. inui, and P. knowlesi, have been implicated in symptomatic malaria in humans in experimental, accidental, or natural infections (2–7). Before the advent of molecular tools for diagnosing infectious diseases, identifying simian malaria in humans required expertise in the structure of these parasites, experimental studies in mosquito vectors, and tests for infectivity to primate hosts (6,7). In general, simian malaria is not included in the differential diagnosis of human infections, which could partly stem from lack of awareness about the zoonotic potential of these parasites. Furthermore, the current laboratory methods for species differentiation target only the four human plasmodia species. On the other hand, simian malaria species that display structural similarity to those species commonly found in humans may be unnoticed in routine examinations of blood smears. We describe a patient who acquired P. knowlesi infection while staying in a forest in southern Thailand where human malaria is endemic.
In August 2000, a 38-year-old Thai man came to an outpatient department of King Chulalongkorn Memorial Hospital, Bangkok, with daily fever, headache, intermittent chill, sweating, and malaise for 4 days. His home was in a suburb of Bangkok, where no malaria transmission has been reported. During the past few months before the present illness, he spent several few weeks in a hilly forest area in Prachuap Khiri Khan Province in southern Thailand, ≈300 km from Bangkok near the Thai-Myanmar border. He reported having fever 1 week after returning home. He did not know of any underlying illness and had not experienced any previous malaria attacks. Although he stayed in a cottage and slept inside a mosquito net, he remembered being bitten frequently by mosquitoes, especially at dusk and dawn.
Upon examination, his temperature was 38.5°C, and pulse rate was 90 beats per minute. His hemoglobin was 14.0 g/dL, hematocrit was 0.4, and erythrocyte count was 4.2 x 106 cells/μL. The total leukocyte count was 5,500 cells/μL, with normal differential count. The platelet count was 90,000/μL. Levels of other laboratory investigations, including urinalysis, blood sugar, liver function test, blood urea nitrogen, and creatinine, were normal. Examination of Giemsa-stained thin blood films showed 10% young trophozoites, 45% growing trophozoites, 40% schizonts, and 5% gametocytes (n = 300). The parasite structure was compatible with that of P. malariae. The parasite density inferred from the number of malarial parasites per 500 leukocytes in thick blood smear yielded 1,155/μL or equivalent to parasitemia 0.03%. The patient was treated with 10 mg/kg of oral chloroquine initially, followed by 5 mg/kg, 6 hours later on the day 1, and 5 mg/kg/day for the next 2 days. On day 2, with a temperature of 37.5°C, he came to the hospital. Parasitemia decreased to 137/μL. Complete defervescence was observed on day 3, and parasitemia could not be detected. Two weeks and 2 months later, his blood smears were negative for malaria. Fever did not recur.
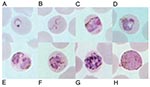
Meanwhile, we recently evaluated a DNA-based diagnostic method by the polymerase chain reaction (PCR) targeting the small subunit ribosomal RNA (SSU rRNA) genes of all four species of human malaria as reported (8). Ten isolates each for P. falciparum, P. vivax, and P. malariae and four isolates of P. ovale were used as positive controls. Results showed that all isolates gave concordant positive PCR products with those diagnosed by microscopy except an isolate from this patient (data not shown). Retrospective examination of blood smears has shown several developmental stages of malaria parasites similar to those typically seen in P. malariae. However, some erythrocytes that harbored mature asexual parasites possessed fimbriated margins. The cytoplasm of some young trophozoites appeared spread out into the network of irregular pseudopodia, and the chromatin was distributed into fragments, conforming to the tenue forms. Pinkish dots varying from fine to large irregular masses called Sinton and Mulligan’s stippling developed intracorpuscularly with the maturation of some parasites (Figure 1).
To elucidate the species of malaria infecting our patient, we determined the SSU rRNA gene by using similar methods as described by others (9), except that ExTaq DNA polymerase (Takara, Japan), pGEM-T vector (Promega, USA), and Escherichia coli strain JM109 were used. Results showed that the SSU rRNA sequence contained 97.8% to 99.6% homology with those of P. knowlesi transcribed during asexual stages or the type A gene (GenBank accession no. AY327549-AY327557, L07560, and U72542) (3,9). Nucleotide sequence data reported in this study are available in the EMBL, GenBank, and DDJB databases under the accession no. AY580317–8.
Phylogenetic tree showed that P. knowlesi in this study was closely related to the W1 and Nuri strains, although its divergence from Malaysian human isolates was not supported by bootstrap analysis (Figure 2). Consistently, the mitochondrial cytochrome b gene of this isolate, determined by the methods similar to previous report except the PCR primers (mtPk-F:5′-AGGTATTATATTCTTTATACAAATATTAAC-3′ and mtPk-R:5′-TCTTTTATAATGAACAAGTGTAAATAATC-3′), displayed perfect sequence identity with that of P. knowlesi strain H from monkey (AF069621) (4).
P. knowlesi is prevalent among crab-eating macaques, Macaca fascicularis, in the Malaysian peninsula and the Philippines (2,10). Other known natural hosts include pig-tailed macaques, M. nemestrina, and leaf monkeys, Presbytis melalophos (2,10). Although in 1932, Knowles et al. (11) had shown that P. knowlesi isolated from monkey could be infectious to humans, the first naturally acquired human infection with P. knowlesi was not reported until 1965 (6); the patient was infected in a Malaysian forest. In 1971 the second case, albeit presumptive, occurred in a man who also acquired the infection in a forest in Malaysia (12). Recently, a large cluster of human infections caused by P. knowlesi has been identified from Malaysian Borneo (9). Our report has expanded the geographic range for natural transmission of P. knowlesi to a forest in Thailand near southern Myanmar border, where wild populations of crab-eating macaques, despite being considered endangered, are still substantial.
The prevalence of naturally acquired primate malaria in humans can be underestimated from examination of blood films. The reported abundance of ring stages of P. knowlesi found in the first naturally acquired human case led to the initial diagnosis of P. falciparum, while the mature parasites could masquerade as those of P. malariae, as we encountered in this patient (6). Although structural descriptions of young trophozoites of P. knowlesi have been delineated, we were unable to find the ring form with double chromatin dots (9). Conversely, a few young trophozoites resembled the tenue forms, proposed by Stephens in 1914 (13) to be a distinct species. However, the tenue form has recently been recognized to be a P. malariae variant found in Myanmar (8). The presence of the tenue form in the blood smears of our patient, despite the low number, rather suggests a shared structural feature among species of malaria. The possibility of coinfection between P. knowlesi with one or more of the four human malaria species was not supported by our PCR detection. The structure of P. knowlesi is highly dependent on the host erythrocytes, i.e., resembling P. vivax in M. fascicularis, P. falciparum in rhesus monkeys, and P. malariae in humans (2,9,11,12). Although stippling was not seen among P. knowlesi–infected blood smears of Sarawak’s patients, the presence of Sinton-Mulligan stippling in infected erythrocytes in this study is in accord with the report by Fong et al., in which erythrocytic stippling served as one of the diagnostic feature (9,12). Such discrepancy could partly arise from differences in the condition for Giemsa staining, infecting parasite strains, or both.
The complete asexual erythrocytic cycle of P. knowlesi in human and its natural macaque host requires ≈24 hours, coinciding with a quotidian fever pattern. However, fever pattern per se may not be a precise indicator for differentiating malaria caused by P. knowlesi and P. malariae. Although the merogony cycle of P. malariae has been generally known to be 72 hours, fever patterns might not be strictly quartan (14). Meanwhile, the preexisting immunity to P. vivax has reportedly conferred partial resistance to induced infection during malariotherapy (2). Whether naturally acquired immunity against P. vivax can reduce symptoms in P. knowlesi infection requires further investigation.
To date, little is known about the extent of variation in the P. knowlesi population. Analysis of the SSU rRNA gene from the isolate in this study has shown minor difference from those of P. knowlesi from monkeys and patients in Malaysian Borneo (3,9). Evidence from malariotherapy showed that P. knowlesi could lose or increase its virulence on blood passage in humans, which suggests that strain difference could occur in wild populations and might effect humans differently (2). In conclusion, P. knowlesi could contribute to the reemergence of simian malaria in Thailand and southeast Asia, where its vectors, Anopheles leucosphyrus group, are abundant (15).
Dr. Jongwutiwes is a molecular parasitologist and clinician at Department of Parasitology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. His works focus on molecular characterizations of protozoa and helminths of medical importance.
Acknowledgments
We thank the patient for participating in our investigation and S. Obama, M. Kinoshita, K. Komatsu, and M. Charoenkorn for assistance.
This study was funded by the Hitachi Scholarship Foundation and Research Grants from Ministry of Education, Culture, Sports, Science and Technology (Grant No. COE17301-F1) and from Ministry of Health, Labour and Welfare of Japan.
References
- Wolfe ND, Escalante AA, Karesh WB, Kilbourn A, Spielman A, Lal AA. Wild primate populations in emerging infectious disease research: the missing link? Emerg Infect Dis. 1998;4:149–58. DOIPubMedGoogle Scholar
- Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias [original book published 1971] [CD-ROM]. Version 1.0. Atlanta: Centers for Disease Control and Prevention; 2003.
- Waters AP, Higgins DG, McCutchan TF. Evolutionary relatedness of some primate models of Plasmodium. Mol Biol Evol. 1993;10:914–23.PubMedGoogle Scholar
- Escalante AA, Freeland DE, Collins WE, Lal AA. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc Natl Acad Sci U S A. 1998;95:8124–9. DOIPubMedGoogle Scholar
- Bruce-Chwatt LJ. Malaria zoonosis in relation to malaria eradication. Trop Geogr Med. 1968;20:50–87.PubMedGoogle Scholar
- Chin W, Contacos PG, Coatney GR, Kimball HR. A naturally acquired quotidian-type malaria in man transferable to monkeys. Science. 1965;149:865. DOIPubMedGoogle Scholar
- Deane LM, Deane MP, Ferreira Neto J. Studies on transmission of simian malaria and on a natural infection of man with Plasmodium simium in Brazil. Bull World Health Organ. 1966;35:805–8.PubMedGoogle Scholar
- Kawamoto F, Win TT, Mizuno S, Lin K, Kyaw O, Tantulart IS, Unusual Plasmodium malariae-like parasites in southeast Asia. J Parasitol. 2002;88:350–7.PubMedGoogle Scholar
- Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–24. DOIPubMedGoogle Scholar
- Knowles R, Das Gupta BM. A study of monkey-malaria and its experimental transmission to man. Ind Med Gaz. 1932;67:301–20.
- Fong YL, Cadigan FC, Coatney GR. A presumptive case of naturally occurring Plasmodium knowlesi malaria in man in Malaysia. Trans R Soc Trop Med Hyg. 1971;65:839–40. DOIPubMedGoogle Scholar
- Garnham PCC. Malaria parasites and other Haemosporidia. Oxford: Blackwell Scientific Publications;1966.
- McKenzie FE, Jeffery GM, Collins WE. Plasmodium malariae blood-stage dynamics. J Parasitol. 2001;87:626–37. DOIPubMedGoogle Scholar
- Scanlon JE, Peyton EL, Gould DJ. The Anopheles (Cellia) leucosphyrus Donitz 1901 group in Thailand. Proc Pap Annu Conf Calif Mosq Control Assoc. 1967;35:78–83.PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 10, Number 12—December 2004
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Somchai Jongwutiwes, Department of Parasitology, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand; fax 662-252-4963
Top