Volume 11, Number 3—March 2005
Perspective
Fly Transmission of Campylobacter
An annual increase in Campylobacter infection in England and Wales begins in May and reaches a maximum in early June. This increase occurs in all age groups and is seen in all geographic areas. Examination of risk factors that might explain this seasonal increase identifies flies as a potential source of infection. The observed pattern of infection is hypothesized to reflect an annual epidemic caused by direct or indirect contamination of people by small quantities of infected material carried by flies that have been in contact with feces. The local pattern of human illness appears random, while having a defined geographic and temporal distribution that is a function of the growth kinetics of one or more fly species. The hypothesis provides an explanation for the seasonal distribution of Campylobacter infections seen around the world.
Campylobacter spp. are the most common bacterial causes of diarrhea in England and Wales (1). The epidemiologic features of Campylobacter infection have proved difficult to discover, and extensive strain typing has failed to clarify the main transmission routes. Testable hypotheses must be established to explain available evidence, particularly the reason for the observed seasonality. Relatively few outbreaks of Campylobacter gastroenteritis occur (2), and most cases are sporadic. In case-control and case-case studies of sporadic Campylobacter infections, most cases remain unexplained by recognized risk factors (3,4).
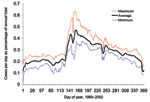
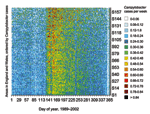
The annual increase in Campylobacter infections in England and Wales begins at approximately day 130 (May 9) and reaches a maximum at approximately day 160 (June 8) (Figure 1). Although this seasonal rise is seen in all ages, it is more marked in children (5). Cases in towns and cities across England and Wales show broadly similar seasonal changes in distribution (Figure 2). The relative geographic uniformity of the increase seen in May of most years has the temporal appearance of an annual national epidemic. Because person-to-person infection within the community is uncommon, it is likely that the epidemic is caused by a single main driver for human Campylobacter infection. The possible seasonal drivers were examined, and only vector transmission by flies appears to provide a convincing explanation for the observed seasonal trends (Table).
The seasonal increase in Campylobacter infections in May and June in England and Wales is hypothesized to reflect an annual epidemic caused by direct or indirect exposure of humans to contaminated material carried by several fly species that have been in contact with human, bird, or animal feces or contaminated raw foods. Flies have been shown to carry Campylobacter and can infect both humans and animals (6–8). Intervention studies have demonstrated diarrheal disease reduction linked to control of flies (9–11), and deaths from diarrheal diseases have been linked to measurements of fly abundance (12). The local pattern of human Campylobacter infection appears random, while having a defined geographic and temporal distribution. This distribution is predicted to be linked to the growth kinetics of 1 or more fly species and their access to environmental sources of Campylobacter in feces or food. The seasonal increase in fly populations results from rainy weather and an increase in temperature that causes the development from egg to fly to occur in days rather than months. Individual flies can lay hundreds of eggs, which can result in a large increase in fly numbers in a short period. Fly numbers fluctuate through the summer and decline in October, but the decline is less dramatic and defined than the spring increase.
Disease transmission is hypothesized to occur through small quantities of contaminated material carried on the feet, proboscis, legs, and body hairs or from material regurgitated or defecated by flies. The variety, numbers, virulence and viability of organisms in the contaminated material will differ, and some contamination will include Campylobacter while others will not. Contamination will be distributed over a variety of food types. Contamination of food by flies could occur at any stage of the food supply chain, but Campylobacter counts within the contaminated material on foods will decrease over time; consequently, most infection will result from contamination close to consumption (e.g., in the domestic or catering environment). Because whether a fly has visited contaminated feces is unknown and how a person becomes infected is uncertain, epidemiologic investigation is difficult.
A number of synanthropic fly species could be involved, including houseflies (e.g., Musca spp., Fannia spp.), blowflies (e.g., Calliphora spp., Lucilia spp.), and other dung-related flies (e.g., Sarcophaga spp., Drosophila spp.) (13). These flies have individual behavioral patterns, ecology, physiology, and temporal and geographic distributions that will influence the likelihood of their being in kitchens, on human or animal feces, and on food. Although Musca domestica is the species most likely to be involved because it is commonly found in houses and food-processing establishments, larger flies (e.g., Calliphora spp.) may be able to transmit larger numbers of Campylobacter.
Flies contaminated through fecal contact will carry heterogeneous mixtures of organisms, including any pathogens that are present within the feces, and may be able to cause a variety of human infections, including infection by different Campylobacter species and types. This fact partially explains the lack of a clear epidemiologic picture arising from Campylobacter typing work. Gastrointestinal disease caused by flies is more likely to involve pathogens with a low infectious dose (e.g., Shigella, Campylobacter, Cryptosporidium, Giardia, Cyclospora, Escherichia coli O157), and some of these could have a seasonal component related to flies. Where high fly populations and poor hygiene conditions prevail, as in disasters or famines, or where pathogens can grow within fly-contaminated food, the potential exists for transmitting pathogens with a high infectious dose (e.g., Vibrio cholerae, Salmonella spp.). The access that flies have to human and animal feces will influence the degree to which they are contaminated with different enteric pathogens.
Contamination of a range of foods by flies will result in a pattern of infection that will not be amenable to identifying specific vehicles through standard case-control, case-case, or cohort studies, unless specific objective or subjective assessments of fly numbers can be obtained. Fly monitoring will need to be undertaken. An alternative approach could use estimates of fly population numbers based on climatic conditions to compare with data on human Campylobacter infections. This approach has the advantage of being able to use historical climatic and disease surveillance data. The broad relationship between Campylobacter cases and ambient temperature has not been explained in terms of disease causation. The time taken for the larvae of M. domestica to develop (13) was applied to temperature data for England and Wales and has been used to show a strong relationship between Campylobacter cases per week and M. domestica larval development time for 1989 to 1999 (Figure 3). Periods when Campylobacter cases exceed a 7-day average of 170 cases per day occurred when M. domestica larval development time was <3 weeks.
The hypothesis predicts that the Campylobacter infection rates will be higher in persons living close to animal production and lower in urban settings because fly numbers will be lower. Some evidence from the United Kingdom (1,14) and Norway (15) supports this hypothesis. Seasonal changes in Campylobacter incidence that are seen around the world may result from changes in fly populations and flies' access to human and animal feces. Much emphasis on foodborne disease reduction has rightly been on kitchen hygiene, since the low infectious dose of Campylobacter makes cross-transmission from raw meats to ready-to-eat foods a substantial risk in domestic and catering environments. Fly transmission may be the most important source of infection in kitchen transmission routes, and establishments that sell ready-to-eat foods may be sources of Campylobacter, if effective fly control is not in operation. Flies may also be important in transmitting Campylobacter in poultry flocks (16) and between other agricultural animals.
While flies are regarded as important mechanical vectors of diarrheal disease in developing countries, control has largely concentrated on improving drinking water and sewage disposal. In the industrialized world, flies are thought to play a minor role in the transmission of human diarrheal diseases. Immediately intervening in the transmission of Campylobacter gastroenteritis should be possible through increased public awareness and more effective fly control.
Supplementary Information
Temperature Data
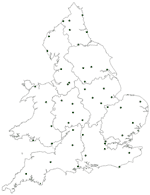
Temperature data were acquired from the British Atmospheric Data Centre (BADC), the Natural Environment Research Council's (NERC) Designated Data Centre for the Atmospheric Sciences based at the Rutherford Appleton Laboratory in Oxfordshire, part of the Central Laboratory of the Research Councils. Data are available on-line through a World Wide Web interface (http://badc.nerc.ac.uk) by prearranged agreement. Data were collated for the period 1989–1999, with 5 locations selected for each region to provide overall coverage of the region (except London, which had only 2 centers with data available for the given time period). Location of temperature stations is shown in the Figure A1.
There were a total of 47 sites. Some of the data series were missing data points. The maximum, minimum, and average temperatures were determined for all days between January 1, 1989, and December 31, 1999. Maximum temperatures across all sites were used to calculate the presumptive minimum Musca domestica larval development times.
Methods
The data represent patients who had fecal specimens examined by a microbiology laboratory in England and Wales between 1989 and 2003 where Campylobacter was isolated from the sample. Data were acquired through well-described surveillance processes, and analysis was conducted in Microsoft Access and Excel (Microsoft Corp., Redmond, WA, USA). Daily cases were based on the patient specimen date, and a 7-day rolling mean was used to eliminate the weekly cycles that reflect reduced patient sampling on weekends.
Hypothesis generation was performed through a systematic review of known and suggested causes of Campylobacter infection, particularly reflecting on changes in these risks over the period of May and June and assessing their credibility as biological drivers for the observed seasonality.
Cities and Towns Included in Figure A1
S1, London; S2, Birmingham; S3, Bristol; S4, Nottingham; S5, Sheffield; S6, Manchester; S7, Leeds; S8, Leicester; S9, Reading; S10, Plymouth; S11, Portsmouth; S12, Colchester; S13, Bradford; S14, Southampton; S15, Poole; S16, Preston; S17, Cardiff; S18, Chelmsford; S19, Norwich; S20, Ipswich; S21, Truro; S22, Oxford; S23, Shrewsbury; S24, Dudley; S25, Taunton; S26, Newport; S27, Cambridge; S28, Newcastle; S29, Chester; S30, Gloucester; S31, Swindon; S32, Chertsey; S33, Coventry; S34, Welwyn; S35, Frimley Park; S36, High Wycombe; S37, Slough; S38, Exeter; S39, Swansea; S40, Luton; S41, Torquay; S42, Derby; S43, York; S44, Worcester; S45, Northampton; S46, Bishops Stortford; S47, Hull; S48, Basildon; S49, Stoke-on-Trent; S50, Worthing; S51, Stafford; S52, Harrogate; S53, Hereford; S54, Halifax; S55, Sunderland; S56, Chesterfield and N Derbyshire; S57, Lincoln; S58, Ashford Kent; S59, Stockport; S60, Blackpool; S61, Maidstone; S62, Liverpool; S63, Bangor; S64, Llandough; S65, Lancaster; S66, Sutton Coldfield; S67, Aylesbury; S68, Grimsby; S69, Doncaster; S70, Peterborough; S71, Brighton; S72, Gateshead; S73, Kettering; S74, Southend; S75, Rhyl; S76, Cheltenham; S77, Epsom; S78, Chichester; S79, Carlisle; S80, Milton Keynes; S81, Dorchester; S82, Durham; S83, Bury; S84, Great Yarmouth; S85, Bury St Edmunds; S86, Warwick; S87, Salisbury; S88, Wolverhampton; S89, Scarborough; S90, Pontefract; S91, Bath; S92, Winchester; S93, Bishop Auckland; S94, Watford; S95, Bolton; S96, Eastbourne; S97, Oldham; S98, North Shields; S99, Burnley; S100, Ashford Middlesex; S101, Kings Lynn; S102, Warrington; S103, Wakefield; S104, Keighley; S105, Crawley; S106, Barnstaple; S107, Abergavenney; S108, Boston; S109, Nuneaton; S110, Northallerton; S111, Wrexham; S112, Macclesfield; S113, Darlington; S114, Bedford; S115, Basingstoke; S116, Weston Supermare; S117, Middlesborough; S118, Dewsbury; S119, Sutton-in-Ashfield; S120, Rochdale; S121, Guildford; S122, Worksop; S123, Wigan; S124, Stevenage; S125, Bridgend; S126, Rotherham; S127, West Bromwich; S128, Solihull; S129, Burton-upon-Trent; S130, Haverford West; S131, Carmarthen; S132, Hemel Hempstead; S133, Stockton-on-Tees; S134, Huddersfield; S135, South Shields; S136, Barnsley; S137, Whitehaven; S138, Chatham; S139, Blackburn; S140, Redditch; S141, St Leonards-on-Sea; S142, Grantham and Kesteven; S143, Ormskirk; S144, Scunthorpe; S145, Canterbury; S146, Kidderminster; S147, Dartford; S148, Aberystwyth; S149, Hexham; S150, Barrow-in Furness; S151, Redhill; S152, Margate; S153, Walsall; S154, Ashington; S155, Salford; S156, Merthyr Tydfil; S157, Stourbridge; S158, Haywards Heath; S159, Banbury; S160, Hartlepool; S161, Prescot; S162, Otley; S163, Southport; S164, Yeovil; S165, Llanelli. The number of reported Campylobacter cases per city and town were based on reports from all laboratories serving the area and are ordered from highest (S1) to lowest (S165) case numbers. Results from towns reporting smaller numbers of cases were excluded from the analysis.
Factors Linked to Campylobacter Infection
The Table A1 provides evidence for seasonal associations between factors linked to human Campylobacter infections or outbreaks.
Dr. Nichols is an epidemiologist in the Communicable Disease Surveillance Centre, which is part of the Health Protection Agency in London. His research interests include waterborne diseases, foodborne diseases, cryptosporidiosis, and enteric infections.
Acknowledgment
This hypothesis arose after a lecture by Professor Sandy Cairncross at the Centers for Disease Control and Prevention, Atlanta, in the spring of 2002. I thank Fay Burgess, Radha Patel, Chris Lane, Douglas Harding, and Erol Yousef for help in preparing the data; Jim McLauchlin, Barry Evans, Chris Little, and John Edmonds for critically commenting on versions of the paper; and André Charlett for statistical support.
References
- Tam CC. Campylobacter reporting at its peak year of 1998: don't count your chickens yet. Commun Dis Public Health. 2001;4:194–9.PubMedGoogle Scholar
- Frost JA, Gillespie IA, O'Brien SJ. Public health implications of campylobacter outbreaks in England and Wales, 1995–9: epidemiological and microbiological investigations. Epidemiol Infect. 2002;128:111–8. DOIPubMedGoogle Scholar
- Adak GK, Cowden JM, Nicholas S, Evans HS. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of campylobacter infection. Epidemiol Infect. 1995;115:15–22. DOIPubMedGoogle Scholar
- Gillespie IA, O'Brien SJ, Frost JA, Adak GK, Horby P, Swan AV, A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses.
- Louis VR, Gillespie IA, O'Brien SJ, Russek-Cohen E, Pearson AD, Colwell RR. Temperature driven Campylobacter seasonality in England and Wales. Appl Environ Microbiol. 2005;71:85–92. DOIPubMedGoogle Scholar
- Khalil K, Lindblom GB, Mazhar K, Kaijser B. Flies and water as reservoirs for bacterial enteropathogens in urban and rural areas in and around Lahore, Pakistan. Epidemiol Infect. 1994;113:435–44. DOIPubMedGoogle Scholar
- Rosef O, Kapperud G. House flies (Musca domestica) as possible vectors of Campylobacter fetus subsp. jejuni. Appl Environ Microbiol. 1983;45:381–3.PubMedGoogle Scholar
- Shane SM, Montrose MS, Harrington KS. Transmission of Campylobacter jejuni by the housefly (Musca domestica). Avian Dis. 1985;29:384–91. DOIPubMedGoogle Scholar
- Chavasse DC, Shier RP, Murphy OA, Huttly SR, Cousens SN, Akhtar T. Impact of fly control on childhood diarrhoea in Pakistan: community-randomised trial. Lancet. 1999;353:22–5. DOIPubMedGoogle Scholar
- Cohen D, Green M, Block C, Slepon R, Ambar R, Wasserman SS, Reduction of transmission of shigellosis by control of houseflies (Musca domestica). Lancet. 1991;337:993–7. DOIPubMedGoogle Scholar
- Emerson PM, Lindsay SW, Walraven GE, Faal H, Bogh C, Lowe K, Effect of fly control on trachoma and diarrhoea. Lancet. 1999;353:1401–3. DOIPubMedGoogle Scholar
- Niven J. Summer diarrhoea and enteric fever. Proc R Soc Med. 1910;III(Epidem. Sect.):131–216.
- Kettle DS. Medical and veterinary entomology. 2nd ed. Wallingford (UK): CABI Publishing; 2000.
- Skirrow MB. A demographic survey of campylobacter, salmonella, and shigella infections in England. A Public Health Laboratory Service survey. Epidemiol Infect. 1987;99:647–57. DOIPubMedGoogle Scholar
- Kapperud G, Aasen S. Descriptive epidemiology of infections due to thermotolerant Campylobacter spp. in Norway, 1979–1988. APMIS. 1992;100:883–90. DOIPubMedGoogle Scholar
- Hald B, Skovgard H, Bang DD, Pedersen K, Dybdahl J, Jespersen JB, Flies and Campylobacter infection of broiler flocks. Emerg Infect Dis. 2004;10:1490–2. DOIPubMedGoogle Scholar
- Allerberger F, Al Jazrawi N, Kreidl P, Dierich MP, Feierl G, Hein I, Barbecued chicken causing a multi-state outbreak of Campylobacter jejuni enteritis. Infection. 2003;31:19–23. DOIPubMedGoogle Scholar
- Evans MR, Lane W, Frost JA, Nylen G. A campylobacter outbreak associated with stir-fried food. Epidemiol Infect. 1998;121:275–9. DOIPubMedGoogle Scholar
- Kessel AS, Gillespie IA, O'Brien SJ, Adak GK, Humphrey TJ, Ward LR. General outbreaks of infectious intestinal disease linked with poultry, England and Wales, 1992–1999. Commun Dis Public Health. 2001;4:171–7.PubMedGoogle Scholar
- Murphy O, Gray J, Gordon S, Bint AJ. An outbreak of campylobacter food poisoning in a health care setting. J Hosp Infect. 1995;30:225–8. DOIPubMedGoogle Scholar
- Pearson AD, Greenwood MH, Donaldson J, Healing TD, Jones DM, Shahamat M, Continuous source outbreak of campylobacteriosis traced to chicken. J Food Prot. 2000;63:309–14.PubMedGoogle Scholar
- Pebody RG, Ryan MJ, Wall PG. Outbreaks of campylobacter infection: rare events for a common pathogen. Commun Dis Rep CDR Rev. 1997;7:R33–7.PubMedGoogle Scholar
- Shandera WX, Tormey MP, Blaser MJ. An outbreak of bacteremic Campylobacter jejuni infection. Mt Sinai J Med. 1992;59:53–6.PubMedGoogle Scholar
- Rodrigues LC, Cowden JM, Wheeler JG, Sethi D, Wall PG, Cumberland P, The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol Infect. 2001;127:185–93. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Outbreak of Campylobacter enteritis associated with cross-contamination of food—Oklahoma, 1996. MMWR Morb Mortal Wkly Rep. 1998;47:129–31.PubMedGoogle Scholar
- Layton MC, Calliste SG, Gomez TM, Patton C, Brooks S. A mixed foodborne outbreak with Salmonella heidelberg and Campylobacter jejuni in a nursing home. Infect Control Hosp Epidemiol. 1997;18:115–21. DOIPubMedGoogle Scholar
- Neal KR, Slack RC. Diabetes mellitus, anti-secretory drugs and other risk factors for campylobacter gastro-enteritis in adults: a case-control study. Epidemiol Infect. 1997;119:307–11. DOIPubMedGoogle Scholar
- Adak GK, Cowden JM, Nicholas S, Evans HS. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of campylobacter infection. Epidemiol Infect. 1995;115:15–22. DOIPubMedGoogle Scholar
- Wilson IG. Salmonella and campylobacter contamination of raw retail chickens from different producers: a six-year survey. Epidemiol Infect. 2002;129:635–45. DOIPubMedGoogle Scholar
- Hanninen ML, Perko-Makela P, Pitkala A, Rautelin H. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J Clin Microbiol. 2000;38:1998–2000.PubMedGoogle Scholar
- Hudson JA, Nicol C, Wright J, Whyte R, Hasell SK. Seasonal variation of Campylobacter types from human cases, veterinary cases, raw chicken, milk and water. J Appl Microbiol. 1999;87:115–24. DOIPubMedGoogle Scholar
- Wallace JS, Stanley KN, Currie JE, Diggle PJ, Jones K. Seasonality of thermophilic Campylobacter populations in chickens. J Appl Microbiol. 1997;82:219–24.PubMedGoogle Scholar
- Humphrey TJ, Henley A, Lanning DG. The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol Infect. 1993;110:601–7. DOIPubMedGoogle Scholar
- Kapperud G, Skjerve E, Vik L, Hauge K, Lysaker A, Aalmen I, Epidemiological investigation of risk factors for campylobacter colonization in Norwegian broiler flocks. Epidemiol Infect. 1993;111:245–55. DOIPubMedGoogle Scholar
- Kirk M, Waddell R, Dalton C, Creaser A, Rose N. A prolonged outbreak of Campylobacter infection at a training facility. Commun Dis Intell. 1997;21:57–61.PubMedGoogle Scholar
- Roels TH, Wickus B, Bostrom HH, Kazmierczak JJ, Nicholson MA, Kurzynski TA, A foodborne outbreak of Campylobacter jejuni (O:33) infection associated with tuna salad: a rare strain in an unusual vehicle. Epidemiol Infect. 1998;121:281–7. DOIPubMedGoogle Scholar
- Ronveaux O, Quoilin S, Van Loock F, Lheureux P, Struelens M, Butzler JP. A Campylobacter coli foodborne outbreak in Belgium. Acta Clin Belg. 2000;55:307–11.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Outbreak of Campylobacter jejuni infections associated with drinking unpasteurized milk procured through a cow-leasing program—Wisconsin, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:548–9.PubMedGoogle Scholar
- Evans MR, Roberts RJ, Ribeiro CD, Gardner D, Kembrey D. A milk-borne campylobacter outbreak following an educational farm visit. Epidemiol Infect. 1996;117:457–62. DOIPubMedGoogle Scholar
- Fahey T, Morgan D, Gunneburg C, Adak GK, Majid F, Kaczmarski E. An outbreak of Campylobacter jejuni enteritis associated with failed milk pasteurisation. J Infect. 1995;31:137–43. DOIPubMedGoogle Scholar
- Jones PH, Willis AT, Robinson DA, Skirrow MB, Josephs DS. Campylobacter enteritis associated with the consumption of free school milk. J Hyg (Lond). 1981;87:155–62. DOIPubMedGoogle Scholar
- Kalman M, Szollosi E, Czermann B, Zimanyi M, Szekeres S, Kalman M. Milkborne campylobacter infection in Hungary. J Food Prot. 2000;63:1426–9.PubMedGoogle Scholar
- Korlath JA, Osterholm MT, Judy LA, Forfang JC, Robinson RA. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis. 1985;152:592–6. DOIPubMedGoogle Scholar
- Lind L, Sjogren E, Melby K, Kaijser B. DNA fingerprinting and serotyping of Campylobacter jejuni isolates from epidemic outbreaks. J Clin Microbiol. 1996;34:892–6.PubMedGoogle Scholar
- Morgan D, Gunneberg C, Gunnell D, Healing TD, Lamerton S, Soltanpoor N, An outbreak of Campylobacter infection associated with the consumption of unpasteurised milk at a large festival in England. Eur J Epidemiol. 1994;10:581–5. DOIPubMedGoogle Scholar
- Porter IA, Reid TM. A milk-borne outbreak of Campylobacter infection. J Hyg (Lond). 1980;84:415–9. DOIPubMedGoogle Scholar
- Robinson DA, Edgar WJ, Gibson GL, Matchett AA, Robertson L. Campylobacter enteritis associated with consumption of unpasteurised milk. BMJ. 1979;1:1171–3. DOIPubMedGoogle Scholar
- Robinson DA, Jones DM. Milk-borne campylobacter infection. Br Med J (Clin Res Ed). 1981;282:1374–6. DOIPubMedGoogle Scholar
- Wood RC, MacDonald KL, Osterholm MT. Campylobacter enteritis outbreaks associated with drinking raw milk during youth activities. A 10-year review of outbreaks in the United States. JAMA. 1992;268:3228–30. DOIPubMedGoogle Scholar
- Lehner A, Schneck C, Feierl G, Pless P, Deutz A, Brandl E, Epidemiologic application of pulsed-field gel electrophoresis to an outbreak of Campylobacter jejuni in an Austrian youth centre. Epidemiol Infect. 2000;125:13–6. DOIPubMedGoogle Scholar
- Djuretic T, Wall PG, Nichols G. General outbreaks of infectious intestinal disease associated with milk and dairy products in England and Wales: 1992 to 1996. Commun Dis Rep CDR Rev. 1997;7:R41–5.PubMedGoogle Scholar
- Public Health Laboratory Service Study Group. Cryptosporidiosis in England and Wales: prevalence and clinical and epidemiological features. BMJ. 1990;300:774–7. DOIPubMedGoogle Scholar
- Riordan T, Humphrey TJ, Fowles A. A point source outbreak of campylobacter infection related to bird-pecked milk. Epidemiol Infect. 1993;110:261–5. DOIPubMedGoogle Scholar
- Stuart J, Sufi F, McNulty C, Park P. Outbreak of campylobacter enteritis in a residential school associated with bird-pecked bottle tops. Commun Dis Rep CDR Rev. 1997;7:R38–40.PubMedGoogle Scholar
- Waldenstrom J, Broman T, Carlsson I, Hasselquist D, Achterberg RP, Wagenaar JA, Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl Environ Microbiol. 2002;68:5911–7. DOIPubMedGoogle Scholar
- Broman T, Palmgren H, Bergstrom S, Sellin M, Waldenstrom J, Danielsson-Tham ML, Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J Clin Microbiol. 2002;40:4594–602. DOIPubMedGoogle Scholar
- Sopwith W, Ashton M, Frost JA, Tocque K, O'Brien S, Regan M, Enhanced surveillance of campylobacter infection in the north west of England 1997–1999. J Infect. 2003;46:35–45. DOIPubMedGoogle Scholar
- Butzler JP, Oosterom J. Campylobacter: pathogenicity and significance in foods. Int J Food Microbiol. 1991;12:1–8. DOIPubMedGoogle Scholar
- Kapperud G. Campylobacter infection. Epidemiology, risk factors and preventive measures. Tidsskr Nor Laegeforen. 1994;114:795–9.PubMedGoogle Scholar
- Ikram R, Chambers S, Mitchell P, Brieseman MA, Ikam OH. A case control study to determine risk factors for campylobacter infection in Christchurch in the summer of 1992–3. N Z Med J. 1994;107:430–2.PubMedGoogle Scholar
- Kapperud G, Skjerve E, Bean NH, Ostroff SM, Lassen J. Risk factors for sporadic Campylobacter infections: results of a case-control study in southeastern Norway. J Clin Microbiol. 1992;30:3117–21.PubMedGoogle Scholar
- Fitzgerald C, Helsel LO, Nicholson MA, Olsen SJ, Swerdlow DL, Flahart R, Evaluation of methods for subtyping Campylobacter jejuni during an outbreak involving a food handler. J Clin Microbiol. 2001;39:2386–90. DOIPubMedGoogle Scholar
- Olsen SJ, Hansen GR, Bartlett L, Fitzgerald C, Sonder A, Manjrekar R, An outbreak of Campylobacter jejuni infections associated with food handler contamination: the use of pulsed-field gel electrophoresis. J Infect Dis. 2001;183:164–7. DOIPubMedGoogle Scholar
- Gent RN, Telford DR, Syed Q. An outbreak of campylobacter food poisoning at a university campus. Commun Dis Public Health. 1999;2:39–42.PubMedGoogle Scholar
- Wight JP, Rhodes P, Chapman PA, Lee SM, Finner P. Outbreaks of food poisoning in adults due to Escherichia coli O111 and campylobacter associated with coach trips to northern France. Epidemiol Infect. 1997;119:9–14. DOIPubMedGoogle Scholar
- Winquist AG, Roome A, Mshar R, Fiorentino T, Mshar P, Hadler J. Outbreak of campylobacteriosis at a senior center. J Am Geriatr Soc. 2001;49:304–7. DOIPubMedGoogle Scholar
- Alary M, Nadeau D. An outbreak of Campylobacter enteritis associated with a community water supply. Can J Public Health. 1990;81:268–71.PubMedGoogle Scholar
- Engberg J, Gerner-Smidt P, Scheutz F, Moller NE, On SL, Molbak K. Water-borne Campylobacter jejuni infection in a Danish town—a 6-week continuous source outbreak. Clin Microbiol Infect. 1998;4:648–56. DOIPubMedGoogle Scholar
- Godoy P, Artigues A, Nuin C, Aramburu J, Perez M, Dominguez A, Outbreak of gastroenteritis caused by Campylobacter jejuni transmitted through drinking water. Med Clin (Barc). 2002;119:695–8.PubMedGoogle Scholar
- Hanninen ML, Haajanen H, Pummi T, Wermundsen K, Katila ML, Sarkkinen H, Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl Environ Microbiol. 2003;69:1391–6. DOIPubMedGoogle Scholar
- Jones IG, Roworth M. An outbreak of Escherichia coli O157 and campylobacteriosis associated with contamination of a drinking water supply. Public Health. 1996;110:277–82. DOIPubMedGoogle Scholar
- Holme R. Drinking water contamination in Walkerton, Ontario: positive resolutions from a tragic event. Water Sci Technol. 2003;47:1–6.PubMedGoogle Scholar
- Maurer AM, Sturchler D. A waterborne outbreak of small round structured virus, campylobacter and shigella co-infections in La Neuveville, Switzerland, 1998. Epidemiol Infect. 2000;125:325–32. DOIPubMedGoogle Scholar
- Melby K, Gondrosen B, Gregusson S, Ribe H, Dahl OP. Waterborne campylobacteriosis in northern Norway. Int J Food Microbiol. 1991;12:151–6. DOIPubMedGoogle Scholar
- Miettinen IT, Zacheus O, von Bonsdorff CH, Vartiainen T. Waterborne epidemics in Finland in 1998–1999. Water Sci Technol. 2001;43:67–71.PubMedGoogle Scholar
- Sacks JJ, Lieb S, Baldy LM, Berta S, Patton CM, White MC, Epidemic campylobacteriosis associated with a community water supply. Am J Public Health. 1986;76:424–8. DOIPubMedGoogle Scholar
- Duke LA, Breathnach AS, Jenkins DR, Harkis BA, Codd AW. A mixed outbreak of cryptosporidium and campylobacter infection associated with a private water supply. Epidemiol Infect. 1996;116:303–8. DOIPubMedGoogle Scholar
- Furtado C, Adak GK, Stuart JM, Wall PG, Evans HS, Casemore DP. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992–5. Epidemiol Infect. 1998;121:109–19. DOIPubMedGoogle Scholar
- Melby KK, Svendby JG, Eggebo T, Holmen LA, Andersen BM, Lind L, Outbreak of Campylobacter infection in a subartic community. Eur J Clin Microbiol Infect Dis. 2000;19:542–4. DOIPubMedGoogle Scholar
- Rautelin H, Koota K, von Essen R, Jahkola M, Siitonen A, Kosunen TU. Waterborne Campylobacter jejuni epidemic in a Finnish hospital for rheumatic diseases. Scand J Infect Dis. 1990;22:321–6. DOIPubMedGoogle Scholar
- Stehr-Green JK, Nicholls C, McEwan S, Payne A, Mitchell P. Waterborne outbreak of Campylobacter jejuni in Christchurch: the importance of a combined epidemiologic and microbiologic investigation. N Z Med J. 1991;104:356–8.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Outbreak of Escherichia coli O157:H7 and Campylobacter among attendees of the Washington County Fair—New York, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:803–5.PubMedGoogle Scholar
- Bopp DJ, Sauders BD, Waring AL, Ackelsberg J, Dumas N, Braun-Howland E, Detection, isolation, and molecular subtyping of Escherichia coli O157:H7 and Campylobacter jejuni associated with a large waterborne outbreak. J Clin Microbiol. 2003;41:174–80. DOIPubMedGoogle Scholar
- Millson M, Bokhout M, Carlson J, Spielberg L, Aldis R, Borczyk A, An outbreak of Campylobacter jejuni gastroenteritis linked to meltwater contamination of a municipal well. Can J Public Health. 1991;82:27–31.PubMedGoogle Scholar
- Aho M, Kurki M, Rautelin H, Kosunen TU. Waterborne outbreak of Campylobacter enteritis after outdoors infantry drill in Utti, Finland. Epidemiol Infect. 1989;103:133–41. DOIPubMedGoogle Scholar
- Merritt A, Miles R, Bates J. An outbreak of Campylobacter enteritis on an island resort, north Queensland. Commun Dis Intell. 1999;23:215–9.PubMedGoogle Scholar
- Said B, Wright F, Nichols GL, Reacher M, Rutter M. Outbreaks of infectious disease associated with private drinking water supplies in England and Wales 1970–2000. Epidemiol Infect. 2003;130:469–79.PubMedGoogle Scholar
- Gillespie IA, O'Brien SJ, Frost JA, Adak GK, Horby P, Swan AV, A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg Infect Dis. 2002;8:937–42.PubMedGoogle Scholar
- Moore J, Caldwell P, Millar B. Molecular detection of Campylobacter spp. in drinking, recreational and environmental water supplies. Int J Hyg Environ Health. 2001;204:185–9. DOIPubMedGoogle Scholar
- Moore JE, Caldwell PS, Millar BC, Murphy PG. Occurrence of Campylobacter spp. in water in Northern Ireland: implications for public health. Ulster Med J. 2001;70:102–7.PubMedGoogle Scholar
- Savill MG, Hudson JA, Ball A, Klena JD, Scholes P, Whyte RJ, Enumeration of Campylobacter in New Zealand recreational and drinking waters. J Appl Microbiol. 2001;91:38–46. DOIPubMedGoogle Scholar
- Hernandez J, Fayos A, Alonso JL, Owen RJ. Ribotypes and AP-PCR fingerprints of thermophilic campylobacters from marine recreational waters. J Appl Bacteriol. 1996;80:157–64. DOIPubMedGoogle Scholar
- Minaev VI, Cherkasskii BL, Volokhovich TT, Minaeva NZ, Pertin OS, Gorelov AV, The leading pathways and factors in the transmission of the causative agents of campylobacteriosis under current conditions. Zh Mikrobiol Epidemiol Immunobiol. 1995; (
2 ):39–42.PubMedGoogle Scholar - Goossens H, Giesendorf BA, Vandamme P, Vlaes L, Van den BC, Koeken A, Investigation of an outbreak of Campylobacter upsaliensis in day care centers in Brussels: analysis of relationships among isolates by phenotypic and genotypic typing methods. J Infect Dis. 1995;172:1298–305. DOIPubMedGoogle Scholar
- Vandamme P, Pugina P, Benzi G, Van Etterijck R, Vlaes L, Kersters K, Outbreak of recurrent abdominal cramps associated with Arcobacter butzleri in an Italian school. J Clin Microbiol. 1992;30:2335–7.PubMedGoogle Scholar
- Morooka T, Umeda A, Fujita M, Matano H, Fujimoto S, Yukitake K, Epidemiologic application of pulsed-field gel electrophoresis to an outbreak of Campylobacter fetus meningitis in a neonatal intensive care unit. Scand J Infect Dis. 1996;28:269–70. DOIPubMedGoogle Scholar
- Evans SJ. The seasonality of canine births and human campylobacteriosis: a hypothesis. Epidemiol Infect. 1993;110:267–72. DOIPubMedGoogle Scholar
- Ellis A, Irwin R, Hockin J, Borczyk A, Woodward D, Johnson W. Outbreak of Campylobacter infection among farm workers: an occupational hazard. Can Commun Dis Rep. 1995;21:153–6.PubMedGoogle Scholar
- Schouls LM, Reulen S, Duim B, Wagenaar JA, Willems RJ, Dingle KE, Comparative genotyping of Campylobacter jejuni by amplified fragment length polymorphism, multilocus sequence typing, and short repeat sequencing: strain diversity, host range, and recombination. J Clin Microbiol. 2003;41:15–26. DOIPubMedGoogle Scholar
- Nielsen EM, Engberg J, Fussing V, Petersen L, Brogren CH, On SL. Evaluation of phenotypic and genotypic methods for subtyping Campylobacter jejuni isolates from humans, poultry, and cattle. J Clin Microbiol. 2000;38:3800–10.PubMedGoogle Scholar
- Savill M, Hudson A, Devane M, Garrett N, Gilpin B, Ball A. Elucidation of potential transmission routes of Campylobacter in New Zealand. Water Sci Technol. 2003;47:33–8.PubMedGoogle Scholar
- Stanley K, Jones K. Cattle and sheep farms as reservoirs of Campylobacter. J Appl Microbiol. 2003;94(Suppl):S104–13. DOIPubMedGoogle Scholar
- Jones K, Howard S, Wallace JS. Intermittent shedding of thermophilic campylobacters by sheep at pasture. J Appl Microbiol. 1999;86:531–6. DOIPubMedGoogle Scholar
- Mann DR, Akinbami MA, Gould KG, Ansari AA. Seasonal variations in cytokine expression and cell-mediated immunity in male rhesus monkeys. Cell Immunol. 2000;200:105–15. DOIPubMedGoogle Scholar
- Stanley KN, Wallace JS, Currie JE, Diggle PJ, Jones K. Seasonal variation of thermophilic campylobacters in lambs at slaughter. J Appl Microbiol. 1998;84:1111–6. DOIPubMedGoogle Scholar
- Stanley KN, Wallace JS, Currie JE, Diggle PJ, Jones K. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J Appl Microbiol. 1998;85:472–80. DOIPubMedGoogle Scholar
- Willis WL, Murray C. Campylobacter jejuni seasonal recovery observations of retail market broilers. Poult Sci. 1997;76:314–7.PubMedGoogle Scholar
- Srour SF, Rishpon S, Rubin L, Warman S. An outbreak of Campylobacter jejuni enteritis after farm visit in Haifa subdistrict. Harefuah. 2002;141:683–4.PubMedGoogle Scholar
- Beecham HJ III, Lebron CI, Echeverria P. Short report: impact of traveler's diarrhea on United States troops deployed to Thailand. Am J Trop Med Hyg. 1997;57:699–701.PubMedGoogle Scholar
- Mattila L, Siitonen A, Kyronseppa H, Simula I, Oksanen P, Stenvik M, Seasonal variation in etiology of travelers' diarrhea. Finnish-Moroccan Study Group. J Infect Dis. 1992;165:385–8. DOIPubMedGoogle Scholar
- Pearson AD, Healing TD. The surveillance and control of campylobacter infection. Commun Dis Rep CDR Rev. 1992;2:R133–9.PubMedGoogle Scholar
- Beecham HJ III, Lebron CI, Echeverria P. Short report: impact of traveler's diarrhea on United States troops deployed to Thailand. Am J Trop Med Hyg. 1997;57:699–701.PubMedGoogle Scholar
- Haberberger RL Jr, Mikhail IA, Burans JP, Hyams KC, Glenn JC, Diniega BM, Travelers' diarrhea among United States military personnel during joint American-Egyptian armed forces exercises in Cairo, Egypt. Mil Med. 1991;156:27–30.PubMedGoogle Scholar
- Black RE. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev Infect Dis. 1990;12(Suppl 1):S73–9. DOIPubMedGoogle Scholar
- Black RE. Pathogens that cause travelers' diarrhea in Latin America and Africa. Rev Infect Dis. 1986;8(Suppl 2):S131–5. DOIPubMedGoogle Scholar
- Brasseur D, Casimir G, Goyens P. Campylobacter jejuni and infantile traveller's diarrhoea. Eur J Pediatr. 1986;144:517–8. DOIPubMedGoogle Scholar
- Echeverria P, Blacklow NR, Sanford LB, Cukor GG. Travelers' diarrhea among American Peace Corps volunteers in rural Thailand. J Infect Dis. 1981;143:767–71. DOIPubMedGoogle Scholar
- Kapperud G, Lassen J, Ostroff SM, Aasen S. Clinical features of sporadic Campylobacter infections in Norway. Scand J Infect Dis. 1992;24:741–9. DOIPubMedGoogle Scholar
- Pazzaglia G, Bourgeois AL, Araby I, Mikhail I, Podgore JK, Mourad A, Campylobacter-associated diarrhoea in Egyptian infants: epidemiology and clinical manifestations of disease and high frequency of concomitant infections. J Diarrhoeal Dis Res. 1993;11:6–13.PubMedGoogle Scholar
- Bichile LS, Saraswati K, Popat UR, Nanivadekar SA, Deodhar LP. Acute Campylobacter jejuni enteritis in 385 hospitalised patients. J Assoc Physicians India. 1992;40:164–6.PubMedGoogle Scholar
- Louis V, Russek-Cohen E, Rubinstein MI, O'Brien SJ, Pearson AD, Colwell RR. Seasonality of Campylobacter cases in England and Wales (1990–1999) [abstract]. Presented at the 103rd General Meeting of the American Society for Microbiology; 2003 May 17–22; Washington, DC.
Figures
Tables
Cite This ArticleTable of Contents – Volume 11, Number 3—March 2005
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Gordon L. Nichols, Environmental and Enteric Diseases Department, Communicable Disease Surveillance Centre, Health Protection Agency Centre for Infections, 61 Colindale Ave, London NW9 5EQ, United Kingdom; fax: 44(0)20-8905-9907
Top