Volume 11, Number 6—June 2005
Research
International Salmonella Typhimurium DT104 Infections, 1992–2001
Cite This Article
Citation for Media
Abstract
The incidence of multidrug-resistant (MDR) Salmonella Typhimurium infections in humans, and in particular MDR definitive phage type 104 (DT104), has increased substantially in many countries in the last 2 decades, often associated with increased illness. To examine the magnitude of this problem, a survey was conducted among countries with available antimicrobial resistance or phage typing surveillance data. A total of 29, primarily industrialized, countries participated in the survey, which covered the years 1992–2001. Overall, the incidence of MDR S. Typhimurium and DT104 increased continuously during this period, although the problem affected primarily Europe and North America. The increase appeared to have peaked in the United Kingdom but not in other countries. Also, the incidence of quinolone-resistant S. Typhimurium was increasing. This survey implies that MDR S. Typhimurium constitutes an increasing public health problem in large parts of the world and emphasizes the importance of surveillance and control programs.
Infections with nontyphoidal Salmonella have increased during the last 3–4 decades, and although a decrease has been reported over the last decade, Salmonella infections continue to be a major public health concern in many countries (1–3). These salmonellae are zoonotic, and the infections are generally foodborne. Although a large number of Salmonella serotypes exist, the overall increase in the number of infections is of relatively few emerging serotypes and phage types. Over periods of several years, certain Salmonella types have risen and (sometimes) fallen within large geographic regions. These meta-outbreaks are facilitated through the acquisition, by specific types, of new traits that make them well adapted to spread, as well as through changes to human society, seen, for instance, with modern intensified farming and food production methods and global trade with live breeder animals (2,4,5). Two prominent examples are the international spread of S. Enteritidis infections through hens eggs (2,6) and the emergence over the last 2 decades of multidrug-resistant (MDR) Salmonella Typhimurium definitive phage type 104 (DT104).
Nontyphoidal Salmonella causes mild to severe, including life-threatening, infections. One study estimated that 600 deaths occur per year in the United States alone due to infections with nontyphoidal Salmonella serotypes (7). A recent study in Denmark showed that infection with nontyphoidal Salmonella was associated with a 2.5-fold increased risk for death within 1 year of infection compared with a matched sample from the general Danish population (8). Salmonellae resistant to antimicrobial drugs appear to pose a particular health risk. Thus, several studies have indicated that infection with salmonellae resistant to ≥1 antimicrobial drugs is associated with increased risk for hospitalization, invasive illness, and death (9–15).
In general, antimicrobial drug resistance occurs frequently in zoonotic salmonellae and is largely promoted by using antimicrobial drugs in food animals (4,16–18). S. Typhimurium DT104 is commonly resistant to 5 drugs: ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline (R-type ACSSuT). S. Typhimurium DT104 was first isolated in the early 1980s in the United Kingdom and later became endemic in bovine animals, from where it spread to the whole food animal production in that country (5,18). Throughout the 1990s, it spread to other parts of the world, and it is now a common Salmonella type in many countries, including the United States, the United Kingdom, Germany, and France (2,19–22). DT104 is common in a broad range of food animals, such as poultry, pigs, and sheep (23). This phage type has become a matter of concern because of its rapid international dissemination in the 1990s and its ability to readily acquire additional resistance traits to other, clinically important antimicrobial drug classes, such as quinolones, trimethoprim, and cephalosporins.
A global survey of salmonellosis and Salmonella serotyping was published in 2002 (24). However, relatively little information has been compiled on the global spread of DT104 and MDR S. Typhimurium (23). Therefore, we have conducted a survey to describe the pandemic of DT104 and MDR S. Typhimurium.
Participating Countries
The survey addressed information on antimicrobial drug resistance testing, phage typing, or both. Since most countries do not routinely apply these typing methods, the questionnaire was not simply sent to all World Health Organization (WHO) member states. Instead, an invitation to participate in the survey was distributed through the WHO Global Salm-Surv (GSS) network (25) and directly to all Enter-Net (26) network countries, plus a group of large countries known or assumed to have resistance testing or phage typing as an integrated part of their national Salmonella surveillance system.
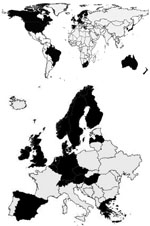
A total of 52 questionnaires were sent out in June and July 2002. Of these, 44 were sent directly to countries known or assumed to have resistance testing or phage typing, and 8 were sent out to member states responding to the GSS invitation. Of the 52 questionnaires, 32 were sent to countries in the European region, 6 to the American Region, 7 to the Asian Region, 5 to the African Region, and 2 to the Oceania Region. Country names and names of geographic regions and subregions are used as described in the United Nations classification system (27).
The Questionnaire
Each country was requested to give information on the total annual number of laboratory-verified episodes of nontyphoidal salmonellosis, S. Typhimurium, MDR S. Typhimurium, and S. Typhimurium DT104 from 1992 to 2001. The participating countries were also asked to give details on whether the reported number of Salmonella isolates that formed the basis for further phage typing or antimicrobial drug susceptibility testing included all national isolates or if they were a subset of isolates. If only a subset of isolates were tested, the countries were asked to state the proportion of S. Typhimurium strains tested and what the criteria for choosing the isolates were. We also asked participants to describe methods used for antimicrobial drug resistance testing and the drugs included in their tests.
Multidrug-resistance was defined as isolates being resistant or intermediately susceptible towards ≥4 separate classes of drugs. This group includes isolates of R-type ACSSuT, the so-called classical penta-resistant phenotype. When information was available, participants were asked to give the numbers of isolates exhibiting R-types extending the ACSSuT-complex and the number of isolates resistant to clinically relevant antimicrobial drugs (quinolones, trimethoprim, and cephalosporins).
In countries where only a subset of Salmonella isolates had been submitted for phage typing, antimicrobial drug-resistance testing, or both, the total number of DT104 or MDR S. Typhimurium isolates was extrapolated from the reported numbers of isolates and the proportion of tested isolates.
The questionnaire was sent to 52 countries, and a completed questionnaire was received from 29, a response rate of 56% (Figure 1). Of the 52 invited countries, 23 were members of or affiliated with Enter-Net; from these a positive feedback was received from 20 (87%). These countries were Australia, Austria, Belgium, Canada, Denmark, England and Wales, Finland, Germany, Greece, Ireland, Japan, Luxembourg, the Netherlands, New Zealand, Norway, Scotland, South Africa, Spain, Sweden, and Switzerland. The 8 countries and 1 regional center (31% response rate) that participated in the survey that were not associated with Enter-Net when data were collected were Brazil, the Czech Republic, Hungary, Israel, Latvia, Malta, Republic of South Korea, United States, and the Caribbean Epidemiology Centre (CAREC), a regional center that represents 21 countries in the Caribbean (Anguilla, Antigua and Barbuda, Aruba, Bahamas, Barbados, Belize, Bermuda, British Virgin Islands, Cayman Islands, Dominica, Grenada, Guyana, Jamaica, Montserrat, Netherlands Antilles, St. Kitts and Nevis, St. Lucia, St. Vincent and the Grenadines, Suriname, Trinidad and Tobago, and Turks and Caicos). For the purpose of this study, CAREC was treated as 1 unit. The 29 participating countries had a total population of 1.028 billion in 2001.
Surveillance
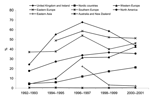
The proportion of Salmonella isolates forwarded from local health laboratories to national or regional institutions varied from 5% to 100%. None of the participating countries restricted the submission of isolates to strains from selected patients, e.g., in case of septicemia or outbreak situations. All 29 participating countries performed serotyping on 85% to 100% of Salmonella isolates received at the national reference laboratory. Table 1 shows the number of nontyphoidal Salmonella isolates and the proportion hereof that were S. Typhimurium in each country from 1992 to 2001 presented as 2-year intervals. The table also shows the estimated incidence of laboratory-confirmed cases of S. Typhimurium in 2001. Throughout the study period, the proportion of S. Typhimurium among nontyphoidal salmonellae has been relatively stable. In 1992, 16% of isolates were S. Typhimurium, compared to 17% in 2001. However, large differences between countries and variations from year to year within each country made comparison difficult. Nevertheless, some trends emerged when the countries were aggregated into geographic regions. In the Caribbean region, South America, Eastern Asia, and Europe, a general decrease in the proportion of S. Typhimurium was observed. In North America, on the other hand, the situation has remained relatively stable, whereas both Australia and New Zealand have seen an increase in the number and proportion of S. Typhimurium cases from 1992 to 2001.
Phage Typing
In 1992, phage typing was carried out in 6 of the participating countries; by 2001, this number had risen to 22. Five countries (Brazil, Japan, Norway, Switzerland, and the United States) only performed phage typing when an MDR strain was found or in outbreak situations. Although not all countries performed phage typing in accordance with the Colindale Scheme (28), the countries using different standards provided information that allowed for comparison with results obtained using the Colindale Scheme (29–31).
The proportion of S. Typhimurium strains that were DT104 and the proportion thereof that were found to be MDR are shown for each country in Table 2. In general, the incidence and proportion of DT104 increased throughout the period. In 1992, 8.7% of S. Typhimurium isolates were DT104, but in 2001 this proportion had increased to 33%. Again, large regional differences occurred. In the United Kingdom, the incidence peaked in 1996 and then decreased. In most other European countries and North America, the relative numbers of DT104 strains had increased throughout the period. In Australia and New Zealand, few DT104 isolates (0.7%) were seen among S. Typhimurium in 2001. The proportion of S. Typhimurium strains that were DT104 is depicted in Figure 2 (the countries are aggregated into 8 regions).
Antimicrobial Drug Resistance
Antimicrobial susceptibility testing became an increasingly common constituent of the surveillance during the study period. In 1992, susceptibility testing was performed in 14 of the 29 participating countries and in all but one in 2000. All countries routinely tested a minimum of 6 antimicrobial drugs, all belonging to different drug classes. The majority of countries (73%) used disc diffusion testing according to NCCLS standards. Listed by frequency, the most common antimicrobial drug classes included in the test panel were (fluoro)quinolones, broad-spectrum penicillins, phenicols, aminoglycosides, tetracyclines, cephalosporins, sulfonamides, and trimethoprim. In 21 countries, antimicrobial susceptibility testing was performed independently of phage typing results, but in 2 countries (Finland until 1999 and New Zealand), testing was only performed on strains found to be DT104.
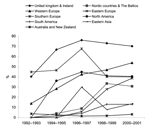
Table 2 shows the distribution of MDR S. Typhimurium presented as 2-year time-bands. Figure 3 depicts the general trend, with countries divided into 9 different regions. In 1992 15% of all S. Typhimurium isolates were MDR; by 2001, this percentage had increased 3-fold to 42%. Once again, large variations occurred between countries and within countries from year to year. In most European countries and North America, MDR S. Typhimurium was common and, with the exception of the United Kingdom and Ireland, multidrug resistance increased from the mid-1990s to the end of the study period. For example, in 2001 multidrug resistance ranged from 22% (Greece) to 72% (Ireland). Only limited data were available from Brazil, the Caribbean region, South Africa, and the Republic of Korea. However, in these regions multidrug resistance was far less common, ranging from zero in the Caribbean region to 19% in the Republic of Korea. In Australia, multidrug resistance remained low throughout the period, with 1.0% of strains in 1997 and 3.6% in 2001.
MDR DT104 was a frequent subtype of MDR S. Typhimurium in most of the countries. In 1992, between 11% (Germany) and 77% (Scotland) of the MDR strains were DT104; in 2001 this ranged from 22% (Australia) to 94% (the Netherlands). Overall, the proportion of MDR DT104 of all DT104 has remained fairly stable; 84% of DT104 were classified as MDR in 2001, compared with 72% in 1992. Among MDR DT104, the classical penta-resistant phenotype, R-type ACSSuT, was by far the most common phenotype throughout the period. It was found in 99% of MDR DT104 strains in 1992 and in 94% of such strains in 2001 (data not shown).
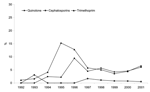
Finally, we looked at trends in development of additional resistance in the classical penta-resistant phenotype (R-type ACSSuT), with focus on 3 clinically important antimicrobial drug classes: quinolones, cephalosporins, and trimethoprim. Both quinolone and trimethoprim resistance increased in MDR DT104 throughout the study period. In 1992, nalidixic acid susceptibility testing was performed on a total of 194 MDR DT104 isolates in 4 different countries; no resistant isolates were found. In 2001, nalidixic acid susceptibility testing of 1,812 MDR DT104 strains in 11 countries identified 109 (6.0%) resistant strains. Similarly, trimethoprim resistance was found in 1.2% of the 180 MDR DT104 strains tested in 1992, but in 6.6% of 1,855 MDR DT104 strains tested in 2001. Cephalosporin-resistant MDR DT104 remained rare, with only 0.5% resistant strains in 2001, and no clear trend observable. Figure 4 shows the overall trend of resistance to quinolone, trimethoprim, and cephalosporins from 1992 to 2001. The increase in quinolone resistance seen in 1996 and in 1998 was caused by a general increase in quinolone-resistant MDR DT104 in Scotland and an outbreak of quinolone-resistant MDR DT104 in Denmark (14), respectively. The increase in trimethoprim resistance from 1995 to 1996 was also caused by a general increase in trimethoprim-resistant MDR DT104 in Scotland.
The present survey was conducted to gain a better understanding of the global impact of DT104 and MDR S. Typhimurium, given the severity of illness than can result from infection with S. Typhimurium and MDR strains in particular. The survey's primary findings are that during the period 1992–2001, the total number of MDR S. Typhimurium and S. Typhimurium DT104 cases increased, while that of other types of S. Typhimurium decreased. The total number of nontyphoidal Salmonella and S. Typhimurium cases also decreased. However, these general findings mask large differences in regional trends. The collected data also may not always accurately describe the real national incidence or be directly comparable between countries.
The total number of isolates of nontyphoidal salmonellae registered at the national level decreased from 1992 to 2001. This result may be biased because of changes in surveillance practices. Since the survey indicated that surveillance systems generally improved throughout the study period, this trend is most likely correct, however. Concurrent with this decrease, an overall decrease in the number of S. Typhimurium isolates was observed, thus keeping the proportion of S. Typhimurium cases among total Salmonella cases constant. The decrease was primarily seen in Europe and North America, whereas Australia and New Zealand saw an increase in both the number of cases of S. Typhimurium and of the total number of nontyphoidal Salmonella cases. In Germany the total number of nontyphoidal Salmonella cases is available for the whole country, but for administrative reasons data on serotypes, antimicrobial susceptibility, and phage types are based only on results from the new federal states of Germany (formerly East Germany) and the city of Berlin. However, German studies have shown that serotype distribution and drug resistance are comparable between former West and East Germany (32) (W. Rabsch, pers. comm.).
MDR S. Typhimurium has increased in the past decades in almost all the regions covered in this study. Most of this increase is due to the concurrent upsurge of DT104, whereas other phage types, such as U302, DT120, DT12, and DT193, were reported to play a smaller role in this development. A high proportion of MDR S. Typhimurium was primarily observed in Europe and North America. Because of the low coverage of participating countries in Asia, South America, and Africa, the situation in these areas was difficult to assess. Both Australia and New Zealand, however, reported high incidence of S. Typhimurium, but MDR strains of S. Typhimurium and DT104 were largely absent. The isolated increase in MDR S. Typhimurium in Australia in 2001 can be explained by a large outbreak of MDR DT104 associated with consumption of halva (dessert made of sesame seeds) (33). That DT104 has not spread markedly in Australia and New Zealand may be explained both by geography and the very strict food and livestock import restrictions in force in these countries, which prevent any large-scale introduction and spread of foreign Salmonella types in the food animal production chain (34).
Although high and generally increasing levels of MDR S. Typhimurium were observed in Europe, the United Kingdom presents a special case. It and Germany had an increase in DT104 in the beginning of the 1990s, before most other countries (5,35). In fact, DT104 was first isolated in the United Kingdom in the early 1980s, years before it was isolated in other countries (5). In the United Kingdom, the incidence of DT104 peaked in 1996 and has since declined ([5] and this study). Possible explanations for this finding include the management of bovine spongiform encephalitis in the United Kingdom, associated general improvements in farm hygiene, and an overall decline in cattle production (36).
Although multidrug-resistance and DT104 were closely linked, large country-specific differences were seen, even between neighboring countries. Most pronounced was the difference between Germany and the Netherlands in 2001, where 64% and 94%, respectively, of MDR isolates were DT104. Such differences probably reflect both real differences and biases resulting from, for instance, different laboratory reporting practices. For example, some of the countries in this survey (New Zealand, Norway, and the United States) performed antimicrobial susceptibility tests on all their DT104 isolates but only on a subset of non-DT104 strains.
In Australia and in Scandinavia, where the incidence of domestically acquired salmonellosis is generally low, many cases appeared to be imported. In a recent study of Australian DT104 isolates, 37% were associated with travel abroad, particularly to Southeast Asia (D. Lightfoot, pers. comm.). Complete data on travel association of MDR S. Typhimurium cases were available for Norway and Finland and showed that most MDR S. Typhimurium patients were infected abroad. In Sweden, where information on DT104 but not MDR was available, most DT104 patients were infected abroad.
A special issue concerns the possibility of acquisition, with time, of resistance traits additional to the classical penta-resistant pattern. Of particular concern is the additional acquisition of quinolone resistance by MDR DT104, since fluoroquinolones are often the drugs of first choice when treating severe salmonellosis. Our data indicate that the prevalence of quinolone resistance has increased. As mentioned, several studies have now shown that multidrug resistance and quinolone resistance may be associated with particular adverse health effects. When seen in the light of the ability of DT104 to spread and establish itself in a large variety of food animal lines (cattle, pigs, poultry), the increase in the number of MDR S. Typhimurium strains that include quinolone resistance becomes particularly problematic. Quinolone, and in particular the fluoroquinolones, have been part of human medicine since the 1980s, resulting in no or very limited resistance in salmonellae. It was not until the license of fluoroquinolones for food animal production in the early 1990s that resistant Salmonella strains emerged (37). The use of fluoroquinolones for food production animals should therefore be discontinued or at least severely restricted as quickly as possible.
This survey has several important limitations. First, it was limited to countries with relatively sophisticated surveillance systems in place, since only countries performing phage typing or resistance testing in addition to serotyping were eligible. This meant that Asia, Africa, and South America were barely covered. Therefore, the survey contains no representative data on the situation in these regions. WHO Global Salm-Surv seeks to enhance the capacity of countries to provide such data. Second, the collection of isolates at the national level is likely to vary from country to country, depending on a number of factors such as sampling frequency at the local level, availability of laboratory reagents, and the degree to which isolates and results are forwarded to the national level. Local practice, the priority given to foodborne illnesses, and financial factors will influence on how often a physician will request a fecal sample. Furthermore, many countries stated that strains and information were not always routinely collected centrally, while in some of the countries strains were never forwarded from certain local laboratories. For these reasons, care should be taken in the interpretation of results of this survey; in particular when comparing incidence rates between countries. However, these limitations are inherent to surveillance in general and do not apply only to this survey. Cross-checking these survey data with available published surveillance data showed them to be in line with each other in the United States (38), the Netherlands (39), and Denmark (40).
In summary, on a global scale, only a small number of countries perform antimicrobial susceptibility testing or phage typing, although the number of countries doing so more than doubled throughout the study period. Despite its limitations, the survey showed that the incidence of both MDR S. Typhimurium and MDR S. Typhimurium DT104 increased markedly worldwide during the 1990s, although the problem has primarily affected Europe and North America. Of special concern is the increasing incidence of quinolone-resistant S. Typhimurium. The survey implies that MDR S. Typhimurium poses a serious and increasing public health problem in large parts of the world. Surveillance and control programs such as the Global Salm-Surv international network recently launched by WHO should therefore be reinforced.
Dr. Helms is a research fellow working on health outcomes in relation to foodborne bacterial infections, in particular, the hazards associated with drug-resistant bacteria in our food supply.
Acknowledgment
We thank WHO Global Salm-Surv for distributing the invitation to participate to members of its network and Wilfrid van Pelt, Ian Fisher, Barbara Foley, and Peter Gerner-Smidt for critical comments on the questionnaire.
References
- Chalker RB, Blaser MJ. A review of human salmonellosis: III. Magnitude of Salmonella infection in the United States. Rev Infect Dis. 1988;10:111–24. DOIPubMedGoogle Scholar
- Tauxe R. Salmonella Enteritis and Salmonella Typhimurium DT104. Successful subtypes in the modern world. In: Emerging infections. Washington: ASM Press; 1999. p. 37–52.
- Gomez TM, Motarjemi Y, Miyagawa S, Kaferstein FK, Stohr K. Foodborne salmonellosis. World Health Stat Q. 1997;50:81–9.PubMedGoogle Scholar
- Swartz MN. Human diseases caused by foodborne pathogens of animal origin. Clin Infect Dis. 2002;34(Suppl 3):S111–22. DOIPubMedGoogle Scholar
- Threlfall EJ, Ward LR, Frost JA, Willshaw GA. Spread of resistance from food animals to man—the UK experience. Acta Vet Scand Suppl. 2000;93:63–8.PubMedGoogle Scholar
- Rodrigues DC, Tauxe RV, Rowe B. International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect. 1990;105:21–7. DOIPubMedGoogle Scholar
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–25. DOIPubMedGoogle Scholar
- Helms M, Vastrup P, Gerner-Smidt P, Mølbak K. Short and long term mortality associated with foodborne bacterial gastrointestinal infections: registry based study. BMJ. 2003;326:357. DOIPubMedGoogle Scholar
- Martin LJ, Fyfe M, Dore K, Buxton JA, Pollari F, Henry B, Increased burden of illness associated with antimicrobial-resistant Salmonella enterica serotype Typhimurium infections. J Infect Dis. 2004;189:377–84. DOIPubMedGoogle Scholar
- Helms M, Simonsen J, Mølbak K. Quinolone resistance is associated with increased risk of invasive illness and death in Salmonella Typhimurium infection. J Infect Dis. 2004;190:1652–4. DOIPubMedGoogle Scholar
- Helms M, Vastrup P, Gerner-Smidt P, Mølbak K. Excess mortality associated with antimicrobial drug-resistant Salmonella Typhimurium. Emerg Infect Dis. 2002;8:490–5.PubMedGoogle Scholar
- Holmberg SD, Wells JG, Cohen ML. Animal-to-man transmission of antimicrobial-resistant Salmonella: investigations of U.S. outbreaks, 1971–1983. Science. 1984;225:833–5. DOIPubMedGoogle Scholar
- Boswell TC, Coleman DJ, Purser NJ, Cobb RA. Development of quinolone resistance in Salmonella: failure to prevent splenic abscess [letter]. J Infect. 1997;34:86–7. DOIPubMedGoogle Scholar
- Mølbak K, Baggesen DL, Aarestrup FM, Ebbesen JM, Engberg J, Frydendahl K, An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica serotype Typhimurium DT104. N Engl J Med. 1999;341:1420–5. DOIPubMedGoogle Scholar
- Varma J, Molbak K, Barrett TJ, Beebe JL, Jones TF, Rabatsky-Ehr T, Antimicrobial-resistant non-typhoidal salmonella is associated with excess bloodstream infections and hospitalizations. J Infect Dis. 2005;191:554–61. DOIPubMedGoogle Scholar
- Cohen ML, Tauxe RV. Drug-resistant Salmonella in the United States: an epidemiologic perspective. Science. 1986;234:964–9. DOIPubMedGoogle Scholar
- Angulo FJ, Johnson KR, Tauxe RV, Cohen ML. Origins and consequences of antimicrobial-resistant nontyphoidal Salmonella: implications for the use of fluoroquinolones in food animals. Microb Drug Resist. 2000;6:77–83. DOIPubMedGoogle Scholar
- Threlfall EJ, Rowe B, Ward LR. A comparison of multiple drug resistance in salmonellas from humans and food animals in England and Wales, 1981 and 1990. Epidemiol Infect. 1993;111:189–97. DOIPubMedGoogle Scholar
- Ward LR, Threlfall EJ, Rowe B. Multiple drug resistance in salmonellae in England and Wales: a comparison between 1981 and 1988. J Clin Pathol. 1990;43:563–6. DOIPubMedGoogle Scholar
- Witte W. Medical consequences of antibiotic use in agriculture. Science. 1998;279:996–7. DOIPubMedGoogle Scholar
- Glynn MK, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo FJ. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–9. DOIPubMedGoogle Scholar
- Rabsch W, Tschape H, Baumler AJ. Non-typhoidal salmonellosis: emerging problems. Microbes Infect. 2001;3:237–47. DOIPubMedGoogle Scholar
- Threlfall EJ. Epidemic Salmonella Typhimurium DT 104-a truly international multiresistant clone. J Antimicrob Chemother. 2000;46:7–10. DOIPubMedGoogle Scholar
- Herikstad H, Motarjemi Y, Tauxe RV. Salmonella surveillance: a global survey of public health serotyping. Epidemiol Infect. 2002;129:1–8. DOIPubMedGoogle Scholar
- Global Salm-Surv [homepage on the Internet]. World Health Organization. 2003 [cited 2005 Mar 31]. Available from www.who.int/salmsurv/en
- Fisher IS. The Enter-Net international surveillance network–how it works. Eurosurveillance. 1999;4:52–5.PubMedGoogle Scholar
- Standard country or area codes for statistical use [monograph on the Internet]. United Nations Statistics Division. 2004 Mar 8 [cited 2005 Mar 31]. Available from http://unstats.un.org/unsd/methods/m49/m49.htm
- Anderson ES, Ward LR, Saxe MJ, de Sa JD. Bacteriophage-typing designations of Salmonella Typhimurium. J Hyg (Lond). 1977;78:297–300. DOIPubMedGoogle Scholar
- Rabsch W. Laborgestützte epidemiologische Analysen–Methodenspektrum und epidemiologische Bewertung. Klassische epidemiologische Laboratoriumsmethoden. In: Kühn H, Tschape H, editors. Salmonellosen des Menschen—Epidemiologische und ätiologische Aspekte. Munich: Medizin Verlag; 1996. p. 118–34.
- van Duijkeren E, Wannet WJ, Houwers DJ, van Pelt W. Antimicrobial susceptibilities of Salmonella strains isolated from humans, cattle, pigs, and chickens in the Netherlands from 1984 to 2001. J Clin Microbiol. 2003;41:3574–8. DOIPubMedGoogle Scholar
- van Duijkeren E, Wannet WJ, Houwers DJ, van Pelt W. Serotype and phage type distribution of Salmonella strains isolated from humans, cattle, pigs, and chickens in the Netherlands from 1984 to 2001. J Clin Microbiol. 2002;40:3980–5. DOIPubMedGoogle Scholar
- Kuehn H. Vorkommen und epidemische Verbreitung. In: Kühn H, Tschäpe H, editors. Salmonellosen des Menschen–Epidemiologische und ätiologische Aspekte. Munich: Medizin Verlag; 1996. p. 19–35.
- Fisher I, Andersson Y, De Jong B, O'Grady KA, Powling J. International outbreak of Salmonella Typhimurium DT104–update from Enter-Net. Eurosurveillance Weekly. 2001;5.
- Crump JA, Murdoch DR, Baker MG. Emerging infectious diseases in an island ecosystem: the New Zealand perspective. Emerg Infect Dis. 2001;7:767–72. DOIPubMedGoogle Scholar
- Prager R, Liesegang A, Rabsch W, Gericke B, Thiel W, Voigt W, Clonal relationship of Salmonella enterica serovar Typhimurium phage type DT104 in Germany and Austria. Zentralbl Bakteriol. 1999;289:399–414. DOIPubMedGoogle Scholar
- Threlfall EJ, Ward LR, Skinner JA, Graham A. Antimicrobial drug resistance in non-typhoidal salmonellas from humans in England and Wales in 1999: decrease in multiple resistance in Salmonella enterica serotypes Typhimurium, Virchow, and Hadar. Microb Drug Resist. 2000;6:319–25. DOIPubMedGoogle Scholar
- Threlfall EJ, Ward LR, Rowe B. Resistance to ciprofloxacin in non-typhoidal salmonellas from humans in England and Wales-the current situation. Clin Microbiol Infect. 1999;5:130–4. DOIPubMedGoogle Scholar
- Salmonella Surveillance CDC. annual summary, 2001. Atlanta: Centers for Disease Control and Prevention; 2002.
- van Pelt W, de Wit MA, Wannet WJ, Ligtvoet EJ, Widdowson MA, van Duynhoven YT. Laboratory surveillance of bacterial gastroenteric pathogens in The Netherlands, 1991–2001. Epidemiol Infect. 2003;130:431–41.PubMedGoogle Scholar
- Annual report on zoonoses in Denmark 2001 [monograph on the Internet]. Ministry of Food, Agriculture and Fisheries. 2002 [cited 2005 Mar 31]. Available from http://www.dfvf.dk/files/filer/zoonosecentret/publikationer/ annual%20report/annual_report_2001_fra_datagraf.pdf
Figures
Tables
Cite This Article1Data for this survey were contributed by the DT104 study group, which consisted of the following: Diane Lightfoot and J. Powling, Australia; Christian Berghold and Christian Kornshober, Austria; Ingrid Wybo, J.M. Collard, and C. Godard, Belgium; Dalia dos Prazeres Rodrigues, Eliane Moura Falavina dos Reis, and Erica L. Fonseca, Brazil; Kathryn Doré, James Flint, Frank Pollari, Rafiq Ahmed, and Walter Demczuk, Canada; James Hospedales, Denise Clarke, and Michelle Nurse-Lucas, Caribbean Region; Renata Karpiskova, The Czech Republic; Peter Gerner-Smidt, Denmark; Noël Gill, Sarah O'Brien, and John Threlfall, England and Wales; Anja Siitonen and Susanna Lukinmaa, Finland; Wolfgang Rabsch, Germany; Panayotis T. Tassios, Leonidas S. Tzouvelekis, and Takis Panagiotopoulos, Greece; Judit Pàszti and Noelni Nógrády, Hungary; Barbara Foley, Martin Cormican, and Paul McKeown, Ireland; Nahum Andorn and Ruti Yishai, Israel; Haruo Watanabe and Hidemasa Izumiya, Japan; Bok Kwon Lee and Shukho Kim, Republic of Korea; J. Selga and J. Jansone, Latvia; Joel Mossong and François Schneider, Luxembourg; Julie Haider and Paul Cuschieri, Malta; Wilfrid van Pelt, Netherlands; Helen Heffernan and Carolyn Nicol, New Zealand; Karin Nygård and Trine Lise Stavnes, Norway; B.L. Cherkasskiy, Russian Federation; Lynda Browning and John Coia, Scotland; Karen Helena Keddy and Tersia Kruger, Republic of South Africa; Miquel Anger Usera, Spain; Agneta Olsson, Sweden; Patrick Boerlin, Switzerland; Timothy Barrett, Frederick J. Angulo, and Jennifer E. Stevenson, USA.
Table of Contents – Volume 11, Number 6—June 2005
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Morten Helms, Department of Epidemiology Research, Statens Serum Institut, Artillerivej 5, DK-2300 Copenhagen S, Denmark; fax: 45-3268-3165
Top