Volume 11, Number 6—June 2005
Dispatch
Cephalosporin and Ciprofloxacin Resistance in Salmonella, Taiwan
Cite This Article
Citation for Media
Abstract
We report the prevalence and characteristics of Salmonella strains resistant to ciprofloxacin and extended-spectrum cephalosporins in Taiwan from January to May 2004. All isolates resistant to extended-spectrum cephalosporins carried blaCMY-2, and all ciprofloxacin-resistant Salmonella enterica serotype Choleraesuis isolates were genetically related.
Resistance to extended-spectrum cephalosporins (ESCs) or fluoroquinolones in Salmonella enterica has become a global concern (1). ESC resistance in Salmonella strains is usually due to the production of plasmid-mediated extended-spectrum β-lactamases (ESBLs) or AmpC β-lactamases, and among these β-lactamases, the CMY-2 AmpC enzyme has been reported most often (1–3). Resistance to fluoroquinolones in Salmonella strains is usually due to the accumulation of mutations in the quinolone resistance–determining regions (QRDRs) of DNA gyrase genes (1,4,5). Resistance to both ESCs and fluoroquinolones remains extremely rare in salmonellae.
In Taiwan, increasing resistance to fluoroquinolones and the emergence of CMY-2–producing ESC-resistant strains in salmonellae have been noted (3–6). The emergence of Salmonella strains resistant to both ceftriaxone and ciprofloxacin was reported more recently in Taiwan and may pose a serious therapeutic problem (7,8). We conducted the present study to investigate the prevalence and characteristics of Salmonella strains resistant to ciprofloxacin and ESCs in Taiwan.
From January to May 2004, a total of 600 Salmonella isolates from 585 patients were obtained from 5 medical centers and 14 district hospitals throughout Taiwan; these isolates were serotyped with commercial antisera (Difco, Detroit, MI, USA). The 4 most common serotypes of Salmonella enterica (Enteritidis, Typhimurium, Stanley, and Choleraesuis) accounted for 66.8% of all isolates. Two isolates were untypeable, and the remainder were typed into 42 serotypes (data not shown), which were each represented by 1 to 23 isolates.
MICs of antimicrobial agents were determined by the agar dilution method (9). Resistance to ciprofloxacin (MIC ≥4 μg/mL) was seen in 50 (8.3%) isolates (Table 1); 20 (3.3%) were resistant (MICs ranging from 8 to >64 μg/mL) to ceftazidime, ceftriaxone, cefotaxime, or aztreonam (Table 2); 6 isolates showed decreased susceptibilities to 1 or 2 of the 4 ESCs (MICs 0.5–2 μg/mL); 10 (1.7%) isolates were resistant to both ciprofloxacin and ESCs. S. Choleraesuis had high rates of resistance to ciprofloxacin (84.4%), ESCs (17.8%), and both (17.8%). None of the 26 Salmonella isolates with resistance or decreased susceptibility to ESCs produced ESBL, according to the double-disk synergy method (10). Among the 20 ESC-resistant isolates, 10 isolates were ciprofloxacin-resistant, 4 isolates showed decreased susceptibility to ciprofloxacin (MIC 0.25–1 μg/mL) and resistance to nalidixic acid, and 6 isolates were susceptible to ciprofloxacin and nalidixic acid (Table 2). All 20 ESC-resistant isolates were susceptible to cefepime (MIC <0.03 μg/mL) and imipenem (MIC <1 μg/mL), and 17 isolates were resistant to >1 non–β-lactam agent.
All 20 ESC-resistant isolates expressed a β-lactamase of pI 9.0 by isoelectric focusing (3,11); 11 of these isolates expressed an additional pI 5.4 β-lactamase (Table 2). blaCMY-2 was detected in all ESC-resistant isolates. blaTEM-1 was detected in the 11 isolates with the pI 5.4 β-lactamase by polymerase chain reaction (PCR) and sequence analyses with the primers for the entire blaTEM-related and blaCMY-2-related structural genes (2,3).
The QRDR sequences of gyrA, gyrB, parC, and parE of the 20 ESC-resistant Salmonella isolates were determined by PCR and sequence analyses (5). All 10 ciprofloxacin-resistant isolates showed 2 mutations at the Ser-83 and Asp-87 codons in gyrA and a single mutation at the Ser-80 codon in parC (Table 2). Four isolates with decreased susceptibility to ciprofloxacin had a single mutation at either the Ser-83 or the Asp-87 codon in gyrA. All 20 ESC-resistant isolates showed no mutations in the QRDRs of gyrB and parE.
ESC resistance was transferred from 18 of the 20 ESC-resistant Salmonella isolates to Escherichia coli C600 in the liquid mating-out assay (3,12). All transconjugants showed decreased susceptibilities to the 4 ESCs tested (MICs 16–64 μg/mL) and cefoxitin (MIC 64–128 μg/mL) and were susceptible to all non–β-lactam agents tested. A pI 9.0 vz β-lactamase and blaCMY-2 were detected by isoelectric focusing and PCR assays, respectively, in all transconjugants. Restricted by the endonuclease EcoRI, the 18 transferred plasmids produced 9 major restriction patterns (Figure 1 and Table 2). Patterns E and I were further divided into 4 and 2 subtypes, respectively. blaCMY-2 on the transferred plasmids was demonstrated by Southern hybridization with the blaCMY-2 probe.
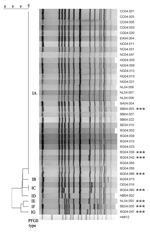
The 38 ciprofloxacin-resistant S. Choleraesuis isolates were genotyped by pulsed-field gel electrophoresis on a CHEF Mapper apparatus (Bio-Rad Laboratories, Hercules, CA, USA) according to the PulseNet protocol (13). Banding patterns generated by XbaI restriction were compared with BioNumerics software (Applied Maths, Kortrijk, Belgium). The 38 isolates showed a close relationship (Dice correlation coefficient of 90%) and had only 1 pulsotype, based on Tenover criteria (Figure 2) (14). The pulsotype was divided into 7 pulsosubtypes, among which were 1–4 band differences. Five ESC-resistant isolates displayed the same pulsosubtypes (IA or IC) as ESC-susceptible isolates (Table 1 and Figure 2).
We describe the prevalence of resistance to ciprofloxacin and ESCs among salmonellae isolated from January to May 2004 in Taiwan. We found widespread resistance of Salmonella isolates to both ESCs and ciprofloxacin; high prevalence of resistance to ciprofloxacin, ESCs, and both in S. Choleraesuis; and widespread prevalence of CMY-2–producing Salmonella isolates of various serotypes in Taiwan.
The prevalence of Salmonella isolates resistant to both ceftriaxone and ciprofloxacin may pose a therapeutic problem. CMY-2 is one of the AmpC enzymes, which are usually less active against cefepime and cefpirome than ESBLs (15). Accordingly, we have used cefepime to successfully treat several patients infected with CMY-2–producing and ciprofloxacin-resistant S. Choleraesuis (8). Therefore, AmpC-producing strains should be differentiated from ESBL-producing strains by phenotypic or genotypic methods when ESC-resistant Salmonella strains are isolated in the clinical microbiology laboratory (15).
The ciprofloxacin-resistant rate in S. Choleraesuis in Taiwan has been >60% since 2001; the high prevalence was mainly due to clonal spread of resistant strains (4–6). The ciprofloxacin-resistant rate in S. Choleraesuis in this report (84.4%) was higher than those reported previously (≤70%) (4–6). blaCMY-2 in Salmonella in Taiwan was first reported in 2 S. Typhimurium strains isolated in 2000 (3). The first reported S. Choleraesuis strain with blaCMY-2 was a ciprofloxacin-resistant strain isolated in 2002 (7). All our 38 ciprofloxacin-resistant S. Choleraesuis isolates, including 8 ESC-resistant isolates, were genetically related. Moreover, we found possibly unrelated blaCMY-2-positive plasmids (lanes 3, 4, 7, 8, 10, and 11 in Figure 1) among closely related isolates (Figure 2). These data together suggest that the development and rapidly increasing prevalence of ESC and ciprofloxacin resistance in S. Choleraesuis in Taiwan might result from the extremely high prevalence of ciprofloxacin resistance followed by the horizontal transfer of blaCMY-2 into ciprofloxacin-resistant epidemic strains rather than from the spread of a clone that had been resistant to ciprofloxacin and ESCs.
All our ciprofloxacin-resistant Salmonella isolates tested had mutations in the QRDRs of gyrA and par, a finding consistent with previously reported results (1,4,5). The rates of ciprofloxacin resistance in the 3 most common serotypes, Enteritidis, Typhimurium, and Stanley, remained very low (0%–0.6%). Six of 11 ciprofloxacin-resistant isolates in the group of uncommon serotypes belonged to serotype Schwarzengrund and accounted for 42.9% of all serotype Schwarzengrund isolates. Thus, the high rate (5.5%) of ciprofloxacin resistance in this group was in part due to the high prevalence of ciprofloxacin resistance in serotype Schwarzengrund.
Dr. Yan is an associate professor, Department of Pathology, National Cheng Kung University College of Medicine, Tainan, Taiwan. His major research interests are the epidemiology and mechanisms of antimicrobial resistance, especially β-lactamases in gram-negative bacteria.
Acknowledgment
This work was partly supported by a research grant NSC93-2320-B-006-016 from the National Science Council, Taiwan.
References
- Su LH, Chiu CH, Chu C, Ou JT. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin Infect Dis. 2004;39:546–51. DOIPubMedGoogle Scholar
- Winokur PL, Brueggemann A, Desalvo DL, Hoffmann L, Apley MD, Uhlenhopp EK, Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob Agents Chemother. 2000;44:2777–83. DOIPubMedGoogle Scholar
- Yan JJ, Ko WC, Chiu CH, Tsai SH, Wu HM, Wu JJ. Emergence of ceftriaxone-resistant Salmonella isolates and the rapid spread of plasmid-encoded CMY-2-like cephalosporinase, Taiwan. Emerg Infect Dis. 2003;9:323–8.PubMedGoogle Scholar
- Chiu CH, Wu TL, Su LH, Chu C, Chia JH, Kuo AJ, The emergence in Taiwan of fluoroquinolone resistance in Salmonella enterica serotype Choleraesuis. N Engl J Med. 2002;346:413–9. DOIPubMedGoogle Scholar
- Hsueh PR, Teng LJ, Tseng SP, Chang CF, Wan JH, Yan JJ, Ciprofloxacin-resistant Salmonella enterica Typhimurium and Choleraesuis from pigs to humans, Taiwan. Emerg Infect Dis. 2004;10:60–8.PubMedGoogle Scholar
- Chiu CH, Wu TL, Su LH, Liu JW, Chu C. Fluoroquinolone resistance in Salmonella enterica serotype Choleraesuis, Taiwan, 2000–2003. Emerg Infect Dis. 2004;10:1674–6.PubMedGoogle Scholar
- Chiu CH, Su LH, Chu C, Chia JH, Wu Tl, Lin TY, et al. Isolation of Salmonella enterica serotype Choleraesuis resistant to ceftriaxone and ciprofloxacin. Lancet. 2004;363:1285–6. DOIPubMedGoogle Scholar
- Ko WC, Yan JJ, Yu WL, Lee HC, Lee NY, Wang LR, A new therapeutic challenge for old pathogens: invasive community-acquired infections caused by ceftriaxone- and ciprofloxacin-resistant Salmonella enterica serotype Choleraesuis. Clin Infect Dis. 2005;40:315–8. DOIPubMedGoogle Scholar
- NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 6th ed. M7-A6. Wayne (PA): The Committee; 2003.
- Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–78. DOIPubMedGoogle Scholar
- Matthew M, Harris M, Marshall MJ, Rose GW. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–78.PubMedGoogle Scholar
- Provence DL, Curtiss R III. Gene transfer in gram-negative bacteria. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR, editors. Methods for general and molecular bacteriology. Washington: American Society for Microbiology; 1994. p. 319–47.
- Graves LM, Swaminathan B. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int J Food Microbiol. 2001;65:55–62. DOIPubMedGoogle Scholar
- Tenover FC, Arbeit R, Goering RV, Mickelsen PA, Murray BE, Persing DH, Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9.PubMedGoogle Scholar
- Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type β-lactamases. Antimicrob Agents Chemother. 2002;46:1–11. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 11, Number 6—June 2005
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Jiunn-Jong Wu, Department of Medical Laboratory Science and Biotechnology, College of Medicine, National Cheng Kung University, No.1 University Rd, Tainan, Taiwan 70101; fax: 886-6-2363956
Top