Volume 11, Number 6—June 2005
Research
Integrating Escherichia coli Antimicrobial Susceptibility Data from Multiple Surveillance Programs
Figure 2
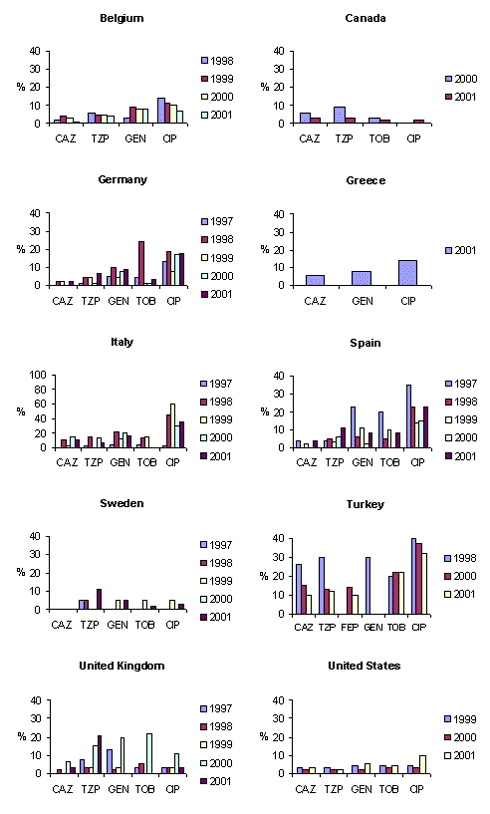
Figure 2. . MYSTIC results for comparison countries. Annual nonsusceptibility rates of Escherichia coli isolates, 1997–2001. p<0.05. CAZ, ceftazidime; TZP, piperacillin/tazobactam; GEN, gentamicin; CIP, ciprofloxacin; TOB, tobramycin; FEP, cefepime.
Page created: April 24, 2012
Page updated: April 24, 2012
Page reviewed: April 24, 2012
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.