Volume 11, Number 6—June 2005
Research
Integrating Escherichia coli Antimicrobial Susceptibility Data from Multiple Surveillance Programs
Cite This Article
Citation for Media
Abstract
Collaboration between networks presents opportunities to increase analytical power and cross-validate findings. Multivariate analyses of 2 large, international datasets (MYSTIC and SENTRY) from the Global Advisory on Antibiotic Resistance Data program explored temporal, geographic, and demographic trends in Escherichia coli resistance from 1997 to 2001. Elevated rates of nonsusceptibility were seen in Latin America, southern Europe, and the western Pacific, and lower rates were seen in North America. For most antimicrobial drugs considered, nonsusceptibility was higher in isolates from men, older patients, and intensive care unit patients. Nonsusceptibility to ciprofloxacin was higher in younger patients, rose with time, and was not associated with intensive care unit status. In univariate analyses, estimates of nonsusceptibility from MYSTIC were consistently higher than those from SENTRY, but these differences disappeared in multivariate analyses, which supports the epidemiologic relevance of findings from the 2 programs, despite differences in surveillance strategies.
The World Health Organization (WHO) highlights the establishment of "effective, epidemiologically sound surveillance of antimicrobial resistance among common pathogens in the community, hospitals, and other health care facilities" as 1 of 2 fundamental public health priorities in efforts to confront antimicrobial drug–resistant organisms (1). At present, most data published in the international literature on antimicrobial resistance are derived from short-term surveys of specific organisms and agents in defined areas. Consequences of this nonsystematic, discontinuous approach are the inability to establish meaningful baseline trends; low sensitivity in detecting new threats; inadequate information to evaluate interventions; and lack of data on organisms, antimicrobial drugs, and patient populations not included in the surveys.
Surveillance groups must coordinate efforts to provide the broadest set of data to policymakers and researchers and to assess the reliability of findings from individual systems. Recognizing the urgency of the problem and the value of joint surveillance collaborations, the Alliance for the Prudent Use of Antibiotics (APUA), a nonprofit organization, established the Global Advisory for Antibiotic Resistance Data (GAARD) (2) in 1999 to involve several of the world's largest multinational enterprises tracking global trends in resistance as well as the Centers for Disease Control and Prevention, WHO, and the WHO Collaborating Centre for Surveillance of Antimicrobial Resistance, which serve in advisory roles. Currently, AstraZeneca International (supporting the Meropenem Yearly Susceptibility Test Information Collection [MYSTIC] surveillance project), Bayer AG (TARGETed), Bristol-Myers Squibb Company (SENTRY), GlaxoSmithKline (Alexander Project), and Ortho-McNeil Pharmaceuticals (TRUST) work with APUA to provide data for GAARD studies. In 2002, data were collected from then-participating GAARD members on Streptococcus pneumoniae (3), Haemophilus influenzae (4), and Escherichia coli. The focus of this article is the analysis of submitted E. coli results from GAARD-participating systems tracking E. coli at that time, i.e., MYSTIC and SENTRY.
E. coli is the most common cause of infections by gram-negative bacilli (5) and the bacterial organism most often isolated from blood cultures (6–9). It is a frequent cause of outpatient urinary tract infections in women worldwide, of hospitalization due to pyelonephritis and septicemia, and of nosocomial infections among hospitalized patients. Meningitis caused by E. coli in neonates is frequently fatal. Resistance to recommended first- and second-line agents, such as penicillins, cephalosporins, sulfa drugs (5,7,10), and fluoroquinolones (11,12) is high in many countries and is commonly associated with treatment failure (13,14).
Antimicrobial susceptibility data on E. coli collected by the MYSTIC and SENTRY systems were forwarded to GAARD coordinators at APUA for descriptive and inferential analysis of temporal, demographic, and geographic trends. MYSTIC was launched by AstraZeneca in 1997 to study bacterial resistance in specialist and general hospital units in hospitals using meropenem (15). At present, 52 sites from 19 countries are contributing results. Each center isolates up to 100 gram-positive and 100 gram-negative aerobic bacteria per year from routine diagnostic samples from hospitalized patients, excluding repeat patient isolates. Antimicrobial susceptibility tests are performed by broth microdilution by using NCCLS reference methods (16) either on-site (for non-US laboratories) or by a reference laboratory (for US participants). More than 9,000 isolates are processed annually, with at least 9 antimicrobial drugs tested per strain.
Bristol-Myers Squibb established the SENTRY program in 1997 as a global program for the surveillance of resistance in bacterial and fungal populations (17). SENTRY has expanded from 75 sites in 1997 to 94 laboratories in 35 countries in 2003. Bacterial isolates are obtained from diagnostic specimens taken in the course of routine clinical management of both hospitalized and community patients. Each site collects a defined number of consecutively identified strains within a number of distinct protocols, e.g., blood isolates, urine isolates, and respiratory isolates, excluding repeat patient isolates. Strains, including basic patient demographic data, are shipped to a coordinating laboratory for centralized identification and susceptibility testing by broth microdilution panels according to NCCLS reference guidelines (16). Forty-five to 50 antimicrobial drugs are monitored each year, with ≈30 tested per strain; >200,000 strains are processed annually.
Available Data
Data on E. coli from 1997 to 2001 were available from 24 countries from the MYSTIC program (4,818 isolates) and 34 countries from SENTRY (14,819 isolates). Because 20 countries are tested by both systems, this figure represents 38 countries, as shown in Table 1. Numbers in the table indicate the number of centers that contributed data at any point during the 5-year period. Descriptive analyses and multivariate regressions included data from all countries, except when data were insufficient (defined as <30 isolates in 2000 and 2001) (18): MYSTIC data from Bulgaria, Malta, Russia, Switzerland, Hong Kong, and Thailand and SENTRY data from Austria, the Netherlands, Portugal, Russia, Mexico, Uruguay, and China. Data from both networks were available for 16 countries, but direct univariate comparisons of findings between the 2 networks were limited to the 10 countries, shown in Figure 1, with at least 20 isolates in each of the years displayed. These 10 "comparison" countries, principally representing North America and Europe, are Belgium, Canada, Germany, Greece, Italy, Spain, Sweden, Turkey, the United Kingdom, and the United States. The United States provided 17% of the MYSTIC isolates and 46% of the SENTRY isolates.
Antimicrobial Drugs
For E. coli in the MYSTIC project, either 12 (United States isolates) or 11 (non-US isolates) antimicrobial drugs were tested. In SENTRY, 26 antimicrobial drugs were examined. The following 8 compounds were tested by both programs and will be referred to as the core antimicrobial agents for comparisons between the 2 networks: cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, meropenem, piperacillin/tazobactam, and tobramycin. Because a primary objective of this study is to highlight the value in contrasting findings from different surveillance programs, most subsequent regression analyses will focus on these 8 agents.
With the exception of ciprofloxacin, these compounds are primarily administered as second-line therapy to hospitalized patients and not routinely to outpatients. Because monitoring resistance to first-line agents is essential to guide empiric treatment decisions, data from the SENTRY network are also presented for the following compounds not tested by MYSTIC laboratories: amoxicillin/clavulanic acid, ampicillin, nalidixic acid, nitrofurantoin, tetracycline, and trimethoprim/sulfamethoxazole.
Data Analysis
Similar demographic data were available from both systems and included patient country, age, and sex; intensive care unit (ICU) or non-ICU location; and specimen type. Susceptibility test data were recorded as MIC values. Resistant, intermediate, and susceptible categories were interpreted according to 2003 NCCLS guidelines (19). During the period studied, NCCLS breakpoints did not change for the drugs studied. Strains with a clinical interpretation of resistant or intermediate were considered nonsusceptible in further analyses.
Available data on E. coli from 1997 through 2001 were sent in Microsoft Excel (Microsoft Corp., Redmond, WA, USA) format by MYSTIC and SENTRY coordinators to APUA for analysis. For descriptive data analysis, files were imported into WHONET 5.2 (World Health Organization, Geneva, Switzerland) (20). Univariate analyses by chi-square testing and multivariate logistic regressions were carried out with Intercooled STATA v. 7 (StataCorp LP, College Station, TX, USA), with null hypotheses rejected for values of p<0.05 and without correction for multiple comparisons. Age was categorized in 10-year intervals, and countries were categorized by geographic region defined in Table 1.
Univariate Comparison of Surveillance Networks
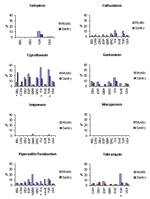
A comparison of the MYSTIC and SENTRY results for 2001 is shown in Figure 1 for the 10 comparison countries. Excluding ciprofloxacin, resistance rates were ≤10% in 2001 for the core antimicrobial drugs among the comparison countries, with the following exceptions: ceftazidime (11.4%) and gentamicin (15.7%) in Italy (MYSTIC); tobramycin (21.9%) in Turkey (MYSTIC); and piperacillin/tazobactam in Spain (10.8%), Sweden (10.9%), Turkey (11.9%), and the United Kingdom (20.9%) (MYSTIC). No isolates confirmed resistant to meropenem or imipenem were found by SENTRY. In the MYSTIC dataset, 2 isolates (from Mexico and Turkey) were found to be nonsusceptible to meropenem and 23 (from Belgium, Brazil, Germany, Mexico, Malta, Turkey, and the United Kingdom) to imipenem. As part of an ongoing protocol for quality assurance, several of these isolates were subsequently confirmed through centralized testing.
Nonsusceptibility estimates in MYSTIC data were consistently higher than in SENTRY. For the 2001 data, country-specific comparisons of MYSTIC to SENTRY nonsusceptibility rates were examined for each antimicrobial drug. From the 46 possible comparisons, MYSTIC estimates were higher than in SENTRY 37 times (80.4%, sign test p<0.001). Excluding comparisons in which either rate was equal to 0%, MYSTIC estimates were on average 2.2 times higher than SENTRY values. Subsequent analysis suggests that the principal contributor to the differences between the surveillance systems would be the higher proportion of ICU patients in MYSTIC (38.0%, n = 1,468) than in SENTRY (19.5%, n = 2,642). Significant differences are depicted in Figure 1.
Univariate Temporal Trends in E. coli Nonsusceptibility
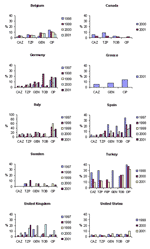
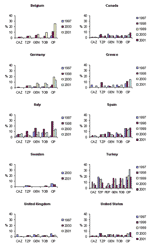
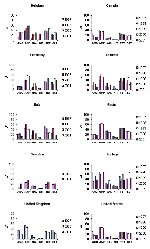
Temporal trends from several of the comparison countries are shown in Figures 2, Figure 3, and 4. With the exception of ciprofloxacin, the antimicrobial drugs tested by both systems are principally reserved for intravenous use in hospitalized patients in most countries, and nonsusceptibility rates for these second-line agents were low worldwide, with some exceptions. Countries with nonsusceptibility rates ≥20% to at least 3 of the core agents by at least 1 of the systems in 2000 or 2001 include Israel, Poland, Mexico, Venezuela, Hong Kong, and the Philippines.
Significant trends (chi-square test for trend without correction for multiple comparisons, p<0.05) evident in the SENTRY dataset include increasing susceptibility to piperacillin/tazobactam in Argentina, Australia, Brazil, Chile, Israel, and the Philippines; increasing susceptibility to cefepime in Argentina and Brazil but decreasing susceptibility in Israel; increasing susceptibility to gentamicin in Brazil and Hong Kong; increasing susceptibility to tobramycin in Australia and Brazil; and decreasing susceptibility to ciprofloxacin in Belgium, Canada, Colombia, and the United States. MYSTIC data showed a significant decreasing trend in nonsusceptibility to ciprofloxacin in Belgium; susceptibility to piperacillin/tazobactam decreased in the United Kingdom; and susceptibility to gentamicin and tobramycin decreased in Israel.
Figure 4 shows trends in nonsusceptibility data in comparison countries for a number of antimicrobial drugs tested only by the SENTRY system commonly prescribed in the outpatient setting. Nonsusceptibility for multiple first-line agents was high (approaching or exceeding 50%) in South Africa, Turkey, Brazil, Chile, Colombia, Venezuela, Hong Kong, the Philippines, Singapore, and Taiwan. Noteworthy trends (p<0.05, chi-square for trends without correction for multiple comparisons) were noted for a number of these agents. Increasing susceptibility to amoxicillin/clavulanic acid was seen in Argentina, Brazil, Canada, Chile, Italy, the United Kingdom, and the United States. Increasing susceptibility to trimethoprim/sulfamethoxazole was seen in Singapore, Chile, Australia, the United States, and Italy, but decreasing susceptibility was seen in Germany; susceptibility to ampicillin decreased in Germany, Colombia, and the Philippines but increased in Chile. Susceptibility to nalidixic acid decreased in Belgium, Canada, Germany, and the United States; susceptibility to nitrofurantoin increased in Canada, Spain, and Chile. Susceptibility to tetracycline increased in Italy and the United Kingdom but decreased in Germany.
Multivariate Trends in E. coli Nonsusceptibility
Multivariate logistic regression was performed to simultaneously control for the effect of potentially confounding variables on nonsusceptibility rates. Independent variables included region, age group, sex, specimen year, ICU specimen source, and surveillance system. Table 2 highlights the significant factors. Because of the rarity of meropenem- and imipenem-resistant isolates in the database, these agents were not studied by logistic regression.
Certain regions (southern Europe, Latin America, and western Pacific), male sex, older age, and ICU isolates were consistently (for at least 4 of the 6 drugs) associated with higher nonsusceptibility rates. North American isolates had lower nonsusceptibility rates (for 5 of the 6 drugs), while isolates from northern Europe had higher rates only for ciprofloxacin. Significant temporal trends were identified only with ciprofloxacin (decreased susceptibility over time, odds ratio [OR] 1.14, 95% confidence interval [CI] 1.07–1.21, p<0.001) and piperacillin/tazobactam (increased susceptibility, OR 0.74, 95% CI 0.68–0.81, p<0.001). For ciprofloxacin, in contrast to findings with other agents, younger age was associated with a higher risk for nonsusceptibility (OR 0.39, 95% CI 0.29–0.52, p<0.001), and nonsusceptibility was not associated with ICU status. An important finding of the multivariate analysis is that the surveillance system (MYSTIC vs. SENTRY) was not associated with nonsusceptibility for any of the compounds, in contrast to the findings of the univariate analyses.
Through integrated analysis of data from multiple sources, the GAARD project seeks to realize a number of benefits: 1) increased statistical power in detecting evolutionary events of public health importance and elucidating risk factors for resistance emergence and spread; 2) greater geographic, demographic, and temporal coverage of bacterial populations than is possible under any single system with limited resources; and 3) cross-validation of findings from complementary data sources with distinct strategies for site recruitment, patient identification, specimen collection, and laboratory testing, which should prompt deeper investigation of seemingly discordant findings (21).
For countries in which a direct comparison of results from the 2 systems was possible, resistance frequencies from MYSTIC were typically higher than from SENTRY. In only 2 instances were higher SENTRY estimates significant (ciprofloxacin in Belgium and Canada). Observation of such incongruent findings should prompt a focused review for possible rationales, such as laboratory testing errors, differences among patient populations sampled, criteria for specimen selection, antimicrobial use patterns, or local outbreaks of resistant organisms. Because SENTRY estimates for Belgium reflect the experience of a single institution while MYSTIC data include results from 9 sites, the MYSTIC results may better reflect the situation in that country.
One of the most substantial findings of the multivariate analysis is that the surveillance system was not associated with nonsusceptibility in any of these compounds, in contrast to the findings of the univariate analyses. Thus, the finding that MYSTIC estimates of nonsusceptibility were consistently higher than SENTRY isolates in paired comparisons may be completely attributable to differences in the demographics of the patient subpopulations sampled. In this study, the principal contributor identified was the proportion of ICU patients represented in the 2 systems. Such findings should increase confidence in the reliability and validity of findings reported separately from the 2 programs. The observation of consistent differences in uncontrolled comparisons of results between systems also highlights the importance of including relevant demographic information in reports on antimicrobial susceptibility rates.
An arbitrary categorization of countries into relatively low, medium, and high resistance is shown in Table 3 for a few key first- and second-line antimicrobial drugs used to treat E. coli infections. The intervals indicated were selected to provide some degree of separation between groups of countries using the observed estimates and should not be interpreted as having a direct clinical implication for therapy decisions. The high rates of resistance to both ampicillin and trimethoprim/sulfamethoxazole in many countries observed in this study should prompt close review of treatment success rates in settings in which they are commonly used in empiric therapy (22).
The use of surveillance data to guide antimicrobial therapy guidelines is a complicated issue that must address the constraints of available resources and therapeutic alternatives, local resistance and antimicrobial use patterns, and potential epidemiologic biases in available data. A number of studies have addressed empiric and quantitative approaches for using surveillance data in treatment guidelines for urinary tract infections and pyelonephritis, including cost-effectiveness studies and establishing resistance thresholds to guide therapy decisions (23–27).
Several significant results were noted in the univariate analyses of temporal trends. Such changes over time could be due to real shifts in the bacterial populations, changes in the number or type of participating institutions, changes in specimen collection practices, or spurious correlations, as no statistical corrections were made for multiple comparisons. The significant decrease to 4 or more agents in Brazil, Chile, and Italy in particular is worth highlighting for further exploration; Chile has successfully implemented and enforced new national legislation banning the sale of antimicrobial drugs without a prescription since 1999, and this legislation has produced substantial reductions in total antimicrobial drug use in the country (28).
Significant findings from the multivariate analysis of core antimicrobial drugs were mentioned above: higher rates of nonsusceptibility in isolates from ICU patients, older patients, and male patients and in isolates from Latin America, the western Pacific, and southern Europe. When all other variables were controlled for, nonsusceptibility to ciprofloxacin showed a statistical increase in over time, while nonsusceptibility to piperacillin/tazobactam decreased. This decrease in nonsusceptibility to piperacillin/tazobactam was significant in 11 countries in univariate analyses and merits further investigation into contributory factors. While temporal trends in the multivariate analysis may reflect, to some degree, the high proportion of US isolates in the SENTRY database, demographic characteristics of SENTRY isolates within and outside the United States were comparable, with only a small but significant difference seen for sex (44.2% [n = 1,058] male in the United States vs. 48.1% [n = 2,331] male outside the United States for 2001 data, p = 0.034).
The higher rate of nonsusceptibility among isolates from male patients has been previously noted for ciprofloxacin resistance (10,12,29) and ascribed to epidemiologic differences between men and women with E. coli infections. Urinary tract infections in male patients are more frequently complicated or healthcare-associated than those in the typical female patient, and infection in men may be associated with higher rates of previous antimicrobial drug usage and time in the hospital setting (29).
The finding of higher resistance in isolates from ICU patients to most agents is not unexpected, given the high selection pressure exerted by intensive antimicrobial use in this setting and the ease of transmission of resistant pathogens on the hands of healthcare workers. The observation that ICU isolates did not have higher rates of resistance to ciprofloxacin, most frequently used in the outpatient setting, suggests that risk factors for ciprofloxacin resistance are distinct from those of the other, principally second-line, agents studied. This dichotomy was also observed with respect to age. For ciprofloxacin, in contrast to the other core antimicrobial drugs, older age was associated with a significant protective effect, i.e., lower nonsusceptibility (OR 0.39, 95% CI 0.29–0.52, p<0.001), than seen in younger patients. One hypothesis holds that resistance in certain antimicrobial drugs, such as intravenous or second-line agents, is more closely associated with patterns of prescribing in hospitals and in older patients, while resistance in others, such as ciprofloxacin, is more correlated with patterns of antimicrobial drug use in the community. This hypothesis merits further investigation in a variety of geographic and clinical settings (30,31). Given the ubiquity of E. coli as a commensal pathogen in the human gut and in animal populations, resistance in E. coli may be a sensitive indicator of distinct therapeutic and nontherapeutic, appropriate and inappropriate uses of antimicrobial drugs (32). Another APUA-coordinated project, Reservoirs on Antibiotic Resistance, is a 5-year scientific collaboration that addresses this issue by exploring the movement of resistance determinants within commensal bacterial populations and between commensals and human pathogens (33).
Both the MYSTIC and SENTRY surveillance networks rely on routinely generated test results, a strategy with advantages over purely research-oriented, resource-intensive special surveys. These advantages include sustainability, more complete organism and geographic coverage, monitoring of baseline trends, infection control alerts, and outbreak detection. However, potential biases may be introduced that must be considered, such as selectively testing patients whose infections did not respond to treatment or who had more severe disease. Such biases may be amplified in the outpatient setting and in low-resource countries where treatment is frequently empiric with limited diagnostic testing. Results from routinely generated sample collections could usefully be compared to findings from periodic validation surveys in which greater resources are expended in identifying and testing representative patient populations (34–36).
With antimicrobial resistance continuing to evolve and present a global public health challenge, appropriately designed and implemented surveillance systems are a priority. Collaboration among existing surveillance systems can improve the quality, breadth, and impact of data for guiding and evaluating clinical and public health policy.
Dr. Stelling is co-director of the WHO Collaborating Center for Surveillance of Antimicrobial Resistance at Brigham and Women's Hospital, Boston, instructor in medicine at Harvard Medical School, and staff scientist at APUA. His research interests include antimicrobial resistance, public health infrastructure for surveillance of resistance and translation of findings into interventions, and development of WHONET and BacLink software tools for the management of microbiology laboratory data.
Acknowledgments
We thank Kathleen Young for her helpful advice in the preparation of this manuscript.
AstraZeneca, Bayer Pharmaceuticals, Bristol-Myers Squibb, and GlaxoSmithKline contributed unrestricted financial support to APUA to operate the GAARD project. Data presented were provided by the SENTRY and MYSTIC programs. APUA is solely responsible for GAARD data analysis and manuscript preparation without industry involvement. The GAARD Project is coordinated by the APUA, Boston, Massachusetts, USA (www.apua.org).
References
- World Health Organization. WHO global strategy for containment of antimicrobial resistance. Geneva: The Organization; 2001.
- Alliance for the Prudent Use of Antibiotics. APUA research and surveillance [homepage on the Internet]. 1999 [cited 2005 Mar 9]. Available from http://www.tufts.edu/med/apua/Research/research.html
- Stelling J, Travers K, Jones R, Miller L, Sahm D, Souder B, Advantages of a coordinated system of independent global surveillance databases: a report from the Global Advisory on Antibiotic Resistance Data (GAARD) [abstract]. International Conference on Emerging Infectious Diseases Program and Abstracts Book; 2002 Mar 24–27. Available from http://www.cdc.gov/iceid/program.htm
- Travers K. Emerging fluoroquinolone resistance [abstract]. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy Abstracts Book; 2002 Sep 27–30; San Diego. Washington: American Society for Microbiology.
- Diekema DJ, Pfaller MA, Jones RN, Doern GV, Winokur PL, Gales AC, Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis. 1999;29:595–607. DOIPubMedGoogle Scholar
- Reacher MH, Shah A, Livermore DM, Wale MC, Graham C, Johnson AP, Bacteraemia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: trend analysis. BMJ. 2000;320:213–6. DOIPubMedGoogle Scholar
- Fluit AC, Jones ME, Schmitz FJ, Acar J, Gupta R, Verhoef J. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin Infect Dis. 2000;30:454–60. DOIPubMedGoogle Scholar
- Vazquez F, Mendoza MC, Viejo G, Mendez FJ. Survey of Escherichia coli septicemia over a six-year period. Eur J Clin Microbiol Infect Dis. 1992;11:110–7. DOIPubMedGoogle Scholar
- Lark RL, Saint S, Chenoweth C, Zemencuk JK, Lipsky BA, Plorde JJ. Four-year prospective evaluation of community-acquired bacteremia: epidemiology, microbiology, and patient outcome. Diagn Microbiol Infect Dis. 2001;41:15–22. DOIPubMedGoogle Scholar
- Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother. 2001;45:1402–6. DOIPubMedGoogle Scholar
- Garau J, Xercavins M, Rodriguez-Carballeira M, Gomez-Vera JR, Coll I, Vidal D, Emergence and dissemination of quinolone-resistant Escherichia coli in the community. Antimicrob Agents Chemother. 1999;43:2736–41.PubMedGoogle Scholar
- Goettsch W, van Pelt W, Nagelkerke N, Hendrix MG, Buiting AG, Petit PL, Increasing resistance to fluoroquinolones in Escherichia coli from urinary tract infections in the Netherlands. J Antimicrob Chemother. 2000;46:223–8. DOIPubMedGoogle Scholar
- Talan DA, Naber KG, Palou J, Elkharrat D. Extended-release ciprofloxacin (Cipro XR) for treatment of urinary tract infections. Int J Antimicrob Agents. 2004;23:54–66. DOIPubMedGoogle Scholar
- Blondeau JM. Current issues in the management of urinary tract infections: extended-release ciprofloxacin as a novel treatment option. Drugs. 2004;64:611–28. DOIPubMedGoogle Scholar
- Turner PJ, Greenhalgh JM, Edwards JR, McKellar J. The MYSTIC (Meropenem Yearly Susceptibility Test Information Collection) programme. Int J Antimicrob Agents. 1999;13:117–25. DOIPubMedGoogle Scholar
- NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—6th ed., M7-A6. Wayne (PA): The Committee; 2003.
- Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997–2002). Diagn Microbiol Infect Dis. 2004;50:59–69. DOIPubMedGoogle Scholar
- NCCLS. Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline, M39-A. Wayne (PA): The Committee; 2002.
- NCCLS. Performance standards for antimicrobial susceptibility testing; 11th informational supplement, M100-S11. Wayne (PA): The Committee; 2001.
- Stelling JM, O'Brien TF. Surveillance of antimicrobial resistance: the WHONET program. Clin Infect Dis. 1997;24:S157–68. DOIPubMedGoogle Scholar
- Finch R. Bacterial resistance—the clinical challenge. Clin Microbiol Infect. 2002;8(Suppl 3):21–32, discussion 3–5. DOIPubMedGoogle Scholar
- Hooton TM, Besser R, Foxman B, Fritsche TR, Nicolle LE. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clin Infect Dis. 2004;39:75–80. DOIPubMedGoogle Scholar
- Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50.PubMedGoogle Scholar
- Graham NM. Epidemiology and pharmacoeconomic issues relating to acute respiratory tract infections and acute uncomplicated infections of the urinary tract. Pharmacoeconomics. 1994;5(Suppl 2):1–10. DOIPubMedGoogle Scholar
- Fenwick EA, Briggs AH, Hawke CI. Management of urinary tract infection in general practice: a cost-effectiveness analysis. Br J Gen Pract. 2000;50:635–9.PubMedGoogle Scholar
- Le TP, Miller LG. Empirical therapy for uncomplicated urinary tract infections in an era of increasing antimicrobial resistance: a decision and cost analysis. Clin Infect Dis. 2001;33:615–21. DOIPubMedGoogle Scholar
- Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis. 1999;29:745–58. DOIPubMedGoogle Scholar
- Bavestrello L, Cabello A, Casanova D. Impact of regulatory measures in the trends of community consumption of antibiotics in Chile [article in Spanish]. Rev Med Chil. 2002;130:1265–72. DOIPubMedGoogle Scholar
- Oteo J, Campos J, Baquero F. Antibiotic resistance in 1962 invasive isolates of Escherichia coli in 27 Spanish hospitals participating in the European Antimicrobial Resistance Surveillance System (2001). J Antimicrob Chemother. 2002;50:945–52. DOIPubMedGoogle Scholar
- Levy SB. Antibiotic and antiseptic resistance: impact on public health. Pediatr Infect Dis J. 2000;19:S120–2. DOIPubMedGoogle Scholar
- Walson JL, Marshall B, Pokhrel BM, Kafle KK, Levy SB. Carriage of antibiotic-resistant fecal bacteria in Nepal reflects proximity to Kathmandu. J Infect Dis. 2001;184:1163–9. DOIPubMedGoogle Scholar
- Working Group on Antibiotic Resistance in Commensal Bacteria. Commensal bacteria—an important reservoir for antibiotic resistance genes? [position paper on the Internet]. Alliance for the Prudent Use of Antibiotics. 1998 Sep [cited 2005 Mar 30]. Available from http://www.tufts.edu/med/apua/ROAR/positionpaper.htm
- Alliance for the Prudent Use of Antibiotics. Reservoirs of Antibiotic Resistance (ROAR) Network [homepage on the Internet]. 2004 [cited 2005 Mar 10]. Available from http://www.tufts.edu/med/apua/ROAR/roarhome.htm
- Livermore DM, Macgowan AP, Wale MC. Surveillance of antimicrobial resistance. Centralised surveys to validate routine data offer a practical approach. BMJ. 1998;317:614–5. DOIPubMedGoogle Scholar
- Heginbothom ML, Magee JT, Bell JL, Dunstan FD, Howard AJ, Hillier SL, Laboratory testing policies and their effects on routine surveillance of community antimicrobial resistance. J Antimicrob Chemother. 2004;53:1010–7. DOIPubMedGoogle Scholar
- Richards J. Monitoring antibiotic resistance in urinary isolates from the community—a spotter practice model. Commun Dis Public Health. 2002;5:226–9.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 11, Number 6—June 2005
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
John M. Stelling, Brigham and Women's Hospital, Microbiology Laboratory, 75 Francis St, Boston, MA 02115, USA; fax: 617-277-1762
Top