Volume 11, Number 8—August 2005
Research
Modeling Control Strategies of Respiratory Pathogens
Figure A6
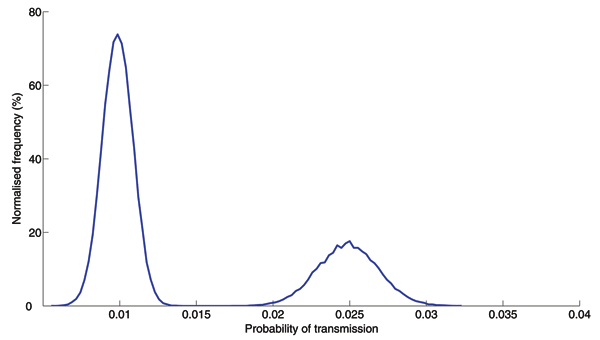
Figure A6. . Transmission probability distribution. The probability of transmission of respiratory pathogens depends on the amount of shedding, distance, duration of contact, and environmental factors such as temperature and humidity. Reports on SARS epidemiology suggest a bimodal distribution of transmission probabilities: close contacts in hospitals may have had high probabilities of transmission while typical contacts in schools, workplaces, and shopping malls may have had low probabilities of transmission (2,3). We assumed this distribution of per day transmission probabilities across the entire network in our analysis. The first peak corresponds to household contacts, while the second peak (with higher probability of transmission) corresponds to all contacts occurring in healthcare settings. Note that this distribution includes the per day probabilities of transmission for all possible contacts in the network, and thus has a smaller mean than the estimates reported for SARS in which the probability of transmission was estimated for particular settings (2,3).
References
- Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. DOIPubMedGoogle Scholar
- Brown H. WHO confirms human-to-human avian flu transmission. Lancet. 2004;363:462. DOIPubMedGoogle Scholar
- Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–50. DOIPubMedGoogle Scholar
- Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–14. DOIPubMedGoogle Scholar
- Normile D. Infectious diseases. First U.S. case of mad cow sharpens debate over testing. Science. 2004;303:156–7. DOIPubMedGoogle Scholar
- Swartz MN. Recognition and management of anthrax—an update. N Engl J Med. 2001;345:1621–6. DOIPubMedGoogle Scholar
- Charatan F. Widespread flu in United States exposes shortage of vaccine. BMJ. 2004;328-a:8.
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford (UK): Oxford University Press; 1991.
- Ferguson NM, Donnelly CA, Anderson RM. The foot-and-mouth epidemic in Great Britain: pattern of spread and impact of interventions. Science. 2001;292:1155–60. DOIPubMedGoogle Scholar
- Pourbohloul B, Rekart ML, Brunham RC. Impact of mass treatment on syphilis transmission: a mathematical modeling approach. Sex Transm Dis. 2003;30:297–305. DOIPubMedGoogle Scholar
- Kaplan EH, Craft DL, Wein LM. Emergency response to a smallpox attack: the case of mass vaccination. Proc Natl Acad Sci U S A. 2002;99:10935–40. DOIPubMedGoogle Scholar
- Meyers LA, Newman ME, Martin M, Schrag S. Applying network theory to epidemics: control measures for Mycoplasma pneumoniae outbreaks. Emerg Infect Dis. 2003;9:204–10.PubMedGoogle Scholar
- Newman MEJ. Spread of epidemic disease on networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;66:016128. DOIPubMedGoogle Scholar
- Longini IM. A mathematical model for predicting the geographic spread of new infectious agents. Math Biosci. 1988;90:367. DOIGoogle Scholar
- Halloran ME, Longini IM Jr, Nizam A, Yang Y. Containing bioterrorist smallpox. Science. 2002;298:1428–32. DOIPubMedGoogle Scholar
- Sattenspiel L, Simon CP. The spread and persistence of infectious diseases in structured populations. Math Biosci. 1988;90:341. DOIGoogle Scholar
- Morris M. Data driven network models for the spread of disease. In: Mollison D, editor. Epidemic models: their structure and relation to data. Cambridge (UK): Cambridge University Press; 1995. p. 302–22.
- Ball F, Mollison D, Scalia-Tomba G. Epidemics with two levels of mixing. Ann Appl Probab. 1997;7:46. DOIGoogle Scholar
- Diekmann O, de Jong MCM, Metz JAJ. A deterministic epidemic model taking account of repeated contacts between the same individuals. J Appl Probab. 1998;35:448–62. DOIGoogle Scholar
- Lloyd AL, May RM. Epidemiology. How viruses spread among computers and people. Science. 2001;292:1316. DOIPubMedGoogle Scholar
- Keeling MJ, Woolhouse MEJ, May RM, Davies G, Grenfell BT. Modeling vaccination strategies against foot-and-mouth disease. Nature. 2003;421:136–42. DOIPubMedGoogle Scholar
- Newman MEJ. The structure and function of complex networks. SIAM Rev. 2003;45:167–256. DOIGoogle Scholar
- Statistics Canada [homepage on the Internet]. [cited 2004 May 6]. Available from http://www.statcan.ca
- BC Statistics [homepage on the Internet]. [cited 2004 May 6]. Available from http://www.bcstats.gov.bc.ca
- Centre for Health Services and Policy Research at University of British Columbia [homepage on the Internet]. [cited May 6]. Available from http://www.chspr.ubc.ca
- City of Vancouver [homepage on the Internet]. [cited 2004 May 6]. Available from http://www.city.vancouver.bc.ca
- Vancouver Public School Board [homepage on the Internet]. [cited 2004 May 6]. Available from http://www.vsb.bc.ca/default.htm
- Diekmann O, Heesterbeek JAP. Mathematical epidemiology of infectious diseases: model building, analysis and interpretation. New York: John Wiley & Sons; 2000.
- Xu R-H, He J-F, Evans MR, Peng G-W, Field HE, Yu D-W, Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004;10:1030–7.PubMedGoogle Scholar
- Yu IT, Li Y, Wong TW, Tam W, Chan AT, Lee JH, Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–9. DOIPubMedGoogle Scholar
- Meyers LA, Pourbohloul B, Newman ME, Skowronski DM, Brunham RC. Network theory and SARS: predicting outbreak diversity. J Theor Biol. 2005;232:71–81. DOIPubMedGoogle Scholar
- Bozzette SA, Boer R, Bhatnagar V, Brower JL, Keeler EB. A model for a smallpox-vaccination policy. N Engl J Med. 2003;348:416–25. DOIPubMedGoogle Scholar
- Chowell G, Fenimore PW, Castillo-Garsow MA, Castillo-Chavez C. SARS outbreaks in Ontario, Hong Kong, and Singapore: the role of diagnosis and isolation as a control mechanism. J Theor Biol. 2003;224:1–8. DOIPubMedGoogle Scholar
- Riley S, Fraser C, Donnelly CA, Ghani AC, Abu-Raddad LJ, Hedley AJ, Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961–6. DOIPubMedGoogle Scholar
- Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L, Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–70. DOIPubMedGoogle Scholar
- Meyers LA, Pourbohloul B, Newman MEJ, Skowronski DM, Brunham RC. Contact networks and the spread of SARS. J Theor Biol. 2005;232:71–81. DOIPubMedGoogle Scholar
- Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–41. DOIPubMedGoogle Scholar
- Donnelly CA, Fisher MC, Fraser C, Ghani AC, Riley S, Ferguson NM, Epidemiological and genetic analysis of severe acute respiratory syndrome. Lancet Infect Dis. 2004;4:672–83. DOIPubMedGoogle Scholar
- Newman MEJ. Spread of epidemic disease on networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;66:016128. DOIPubMedGoogle Scholar
- Meyers LA, Newman MEJ, Pourbohloul B. Predicting epidemics on semi-directed networks. In review. Available from http://www.santafe.edu/research/publications/wplist/2004 (paper #04-12-037).
1These authors contributed equally to this work.
2For the purposes of this manuscript, "airborne" refers to respiratory pathogens that are spread through respiratory secretions and can be either airborne, such as tuberculosis, or dropletborne, such as SARS.