Volume 12, Number 10—October 2006
Research
Active Cytomegalovirus Infection in Patients with Septic Shock
Cite This Article
Citation for Media
Abstract
Cytomegalovirus (CMV) is a pathogen of emerging importance for patients with septic shock. In this prospective study, 25 immunocompetent CMV-seropositive patients with septic shock and an intensive care unit stay of >7 days were monitored by using quantitative pp65-antigenemia assay, shell vial culture, and virus isolation. Within 2 weeks, active CMV infection with low-level pp65-antigenemia (median 3 positive/5 × 105 leukocytes) developed in 8 (32%) patients. Infection was controlled within a few weeks (median 26 days) without use of antiviral therapy. Duration of intensive care and mechanical ventilation were significantly prolonged in patients with active CMV infection. CMV reactivation was associated with concomitant herpes simplex virus reactivation (p = 0.004). The association between active CMV infection and increased illness could open new therapeutic options for patients with septic shock. Future interventional studies are required.
Sepsis and septic shock are defined as a clinical syndrome with severe inflammatory response (1). Despite the availability of antimicrobial, antifungal, and supportive therapies, septic shock is fatal for about one third of patients.
Cytomegalovirus (CMV) is a human β-herpesvirus that has high seroprevalence in adults. CMV disease predominantly occurs as an opportunistic infection in patients with severe immunosuppression and rarely occurs in immunocompetent patients (2). Clinical diagnosis of CMV disease, without the use of virus diagnostics, is hampered by the fact that the clinical signs and symptoms are not very specific. Patients in intensive care units (ICUs) are rarely monitored for active CMV infection; therefore, the development of active CMV infection remains unrecognized in most critically ill patients.
During recent years, CMV has been discussed as an emerging pathogen in critically ill patients who are not receiving immunosuppressive therapy; however, the incidence of active CMV infection is controversial (3,4), and not all centers detected active CMV infections in these patients (5–7). Among critically ill patients, the highest incidence of active CMV infection was in patients with septic shock (3). The causality of sepsis, consecutive CMV reactivation, and CMV-associated pulmonary disease is supported by a mouse model of murine CMV reactivation after cecal ligation and puncture (8,9). Many factors could stimulate CMV reactivation in patients with septic shock; e.g., proinflammatory cytokines (10,11), transient immune paralysis (compensatory antiinflammatory response syndrome) (12), and drugs (13).
This pilot study investigated the incidence and the natural course of active CMV infection in patients with septic shock and different strategies for CMV monitoring. The study may stimulate future interventional trials aimed at preventing CMV-associated illness of patients with septic shock.
Patients
For 9 consecutive months, patients in the anesthesiologic ICU, University Hospital Ulm, Ulm, Germany, who had septic shock, were monitored for active CMV infection. We did not monitor patients who underwent splenectomy, transplantation patients, or patients receiving immunosuppressive therapy. Also, patients who had been in ICU <7 days were excluded because CMV reactivation and CMV-associated illness were expected to develop with a time delay. To define septic shock, we used the criteria established by the American College of Chest Physicians/Society of Critical Care Medicine (14). Clinicians were not made aware of virologic results. To avoid exogenous CMV infections, transfusions were limited to filtered leukocyte-reduced blood products. The study was approved by the local ethics committee and was in accordance with the Helsinki Declaration; informed consent was obtained.
Virologic Examinations
CMV monitoring was performed twice during the first week of the study and once a week thereafter until the patient was discharged from ICU. Quantitative pp65-antigenemia assay; shell vial culture; and viral isolation in leukocytes, urine, and bronchial aspirate were performed as previously described (15). Briefly, pp65 antigenemia was determined in blood collected in EDTA tubes and subjected to dextran sedimentation (1% dextran in phosphate-buffered saline). Duplicates of 5 × 105 leukocytes were placed onto glass slides, and the pp65 antigen-positive cells were evaluated by immunofluorescence assay (IFA) by using a mixture of 2 monoclonal mouse anti-pp65 antibodies (20:1; Virion, Rüschlikon, Switzerland; Argene Biosoft, Viva Diagnostika, Hürth, Germany) and goat anti-mouse immunoglobulin (Ig) G (Dianova, Hamburg, Germany) conjugated with fluorescein isothiocyanate (FITC).
Leukocytes, bronchial aspirate, and urine were investigated by shell vial culture and viral isolation with human embryonic lung fibroblasts. Three days after infection, shell vial cultures were fixed with methanol and analyzed by IFA (anti-CMV immediate early antibody, Argene Biosoft, Viva Diagnostika; FITC-conjugated goat anti-mouse IgG, Dianova). Phase contrast microscopy was used to analyze viral isolation for >6 weeks. Cytopathic effects of various viruses were confirmed by using viral typing with IFA and monoclonal antibodies.
At the initial evaluation, the following antibodies were determined semiquantitatively by using ELISA (Medac, Hamburg, Germany): CMV IgG, CMV IgM, and herpes simplex virus (HSV) IgG. Patients with antibody indices >1 were considered antibody positive.
Clinical Data
The following values were regularly recorded: body temperature, heart rate, blood pressure, respiratory rate, need for mechanical ventilation, oxygen supply (FiO2), urinary output, hemodiafiltration, partial pressure of oxygen in arterial blood, pH, leukocyte count, platelet count, serum bilirubin, aspartate aminotransferase (AST), C-reactive protein, and serum creatinine. The severity of organ failure over time was recorded by monitoring the most relevant organ functions (pulmonary, cardiovascular, hematologic, hepatic) and using the Sepsis-related Organ Failure Assessment Score (SOFA) (16). Impairment of the central nervous system was not evaluated (Glasgow Coma Scale) because most patients received sedatives.
Statistics
Statistical analysis was performed by using nonparametric tests (Fisher exact test, Mann-Whitney U test) and GraphPad Prism 3.02 software (GraphPad Software, San Diego, CA, USA). Significance level was set at p = 0.05.
Patients
Among 375 patients in ICU, 38 consecutive patients with septic shock were eligible, but 13 were excluded because of CMV seronegativity (n = 5), immunosuppressive therapy (n = 2), or ICU stay <7 days (n = 6). Thus, 25 CMV-seropositive patients with septic shock and an ICU stay >7 days were enrolled in the study.
Active CMV Infection
During the first 2 weeks after onset of septic shock, active CMV infection was detected by sensitive quantitative pp65-antigenemia assay in 8 (32%) patients (15). Active CMV infection was also detected by shell vial culture in 4 of these patients (in bronchial aspirate for 3 patients and in urine for 1). For 1 patient for whom shell vial culture in bronchial aspirate was positive, shell vial culture was also positive in leukocytes. Initial detection of active CMV infection was delayed when using shell vial culture (detected 1, 11, 20, and 21 days after onset of septic shock) compared with pp65-antigenemia in the same patients (0, 7, 10, and 14 days). Overall, pp65-antigenemia was low (median 3 positive/5 × 105 leukocytes; range 1–17) and became nondetectable with no antiviral therapy (median 26 days after onset of active CMV infection; range 1–61 days). One patient died while CMV infection was still active.
CMV IgM antibodies were found in 2 (25%) of 8 patients with and 2 (12%) of 17 patients without active CMV infection, a difference that was not significant. Also the quantitative levels of CMV IgG and IgM antibodies did not differ between groups with and without active CMV infection (Table).
Characteristics of Patients with and without Active CMV Infection
Patient characteristics such as age, sex, primary disease, and severity of organ failure at time of entry into the study did not differ between patients with and without active CMV infection (Table). Hydrocortisone (200 mg/day) was given to patients in both groups; no differences between groups were noted in body temperature, leukocyte count, platelet count, serum creatinine level, serum bilirubin level, AST level, and C-reactive protein level. Systemic infection by gram-positive and gram-negative microorganisms was detected equally in both groups, and catecholamine treatment for cardiovascular dysfunction was similar for both groups.
Overall, the severity of sepsis-related failure of multiple organs, determined by SOFA score (16), did not differ between patients with and without active CMV infection; however, patients with active CMV infection required mechanical ventilation and ICU therapy for a longer time than did patients without active CMV infection (p = 0.0025) (Table). Although mortality rates were not significantly different between patients with and without active CMV infection (63% vs 35%; p > 0.05), the deaths occurred later (median 44 days after onset of septic shock, range 24–72 days) for patients with active CMV infection than for patients without (median 21 days, range 14–35 days) (p = 0.03).
The clinical course of patients with positive CMV shell vial culture in bronchial aspirate was associated with the longest duration of mechanical ventilation (47, 50, and 80 days) and of ICU stay (50, 71, and 87 days); however, because of the low number of cases, statistical analysis was not performed.
Other Viral Infections
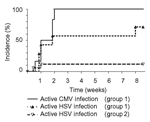
All 25 patients were HSV seropositive; viral isolation in bronchial aspirate showed reactivation of HSV in 8 (32%) patients, thereby showing for the first time that HSV and CMV reactivation were associated (p = 0.004, Table) and occurred simultaneously (Figure). Active HSV infections developed without skin or mucosal rash (occult HSV infection). Because of the low number of cases, the clinical outcome of patients with active CMV, HSV, or CMV/HSV coinfection could not be further differentiated. Viral isolation in bronchial aspirate and urine did not detect additional opportunistic viral infections such as polyoma BK virus and exogenous viral infections such as adenovirus, respiratory syncytial virus, and parainfluenzavirus in any patient.
While CMV is well known as a cause of serious illness in immunosuppressed patients, it is now being discussed as a pathogen of emerging importance for patients with septic shock. Generally, active CMV infection is not recognized in such patients because critically ill patients are not routinely monitored for CMV infection.
CMV reactivation developed in one third of our patients within 2 weeks of onset of septic shock, as has been found in studies using a similar prospective study design (3,11). Diagnostic assays of different sensitivity, different patient groups, and study designs could account for discrepant results obtained by other groups (5,10). Thus, onset of active CMV infection was detected later in the retrospective studies (4,17,18).
Active CMV infection in patients with septic shock was characterized by a low viral load and resolved within a few weeks without antiviral therapy. We hypothesize that upon CMV reactivation, patients with septic shock could mount a protective antiviral immune response, which was different from the immune response of most patients after transplantations (19); however, this hypothesis remains to be confirmed.
In a previous study we compared different assays for CMV monitoring of patients with organ transplants and demonstrated equal sensitivity of our pp65 antigenemia assay and CMV PCR of blood cells but lower sensitivity of shell vial culture, CMV PCR in plasma, and CMV mRNA detection by nucleic acid sequence-based amplification (15). Because of low viral loads, the incidence of active CMV infection could be easily underestimated by less sensitive assays for patients with septic shock, which was shown here in that shell vial culture in blood cells detected only 1 patient with active CMV infection. Less sensitive assays could have been also the problem of studies that failed to detect active CMV infection in critically ill patients (5–7). We assume that assays with sensitivity similar to that of our pp65-antigenemia assay (e.g., CMV PCR of blood cells) may be equally used for CMV monitoring of patients with septic shock, considering the results of patients who had received transplants (3,11,15).
Shell vial culture was more likely to detect active CMV infection in bronchial aspirate than in urine or blood cells. Pulmonary CMV infection may be relevant for patients with septic shock (8,20). Shell vial culture of urine was rarely positive for CMV in patients with septic shock, a finding which differed for patients having received a kidney transplant (21).
As expected, quantitative analysis of CMV IgG and IgM antibodies could not discriminate between patients with and without active CMV infection. CMV IgG antibodies were analyzed to identify patients with previous CMV infection (CMV-seropositive patients); however, diagnosis of active CMV infection by detection of CMV IgM antibodies or rising CMV antibody titers are no longer recommended when sensitive CMV monitoring by pp65-antigenemia assay or CMV PCR are available because the information they provide is limited.
The clinical role of active CMV infection in patients with septic shock is an area of ongoing discussion (4). We demonstrated that active CMV infection is associated with prolonged ventilation time and ICU stay. Ventilation time and ICU stay were more prolonged in a subgroup of patients for whom shell vial culture in bronchial aspirate was positive. CMV infection was associated with pulmonary disease despite low pp65 antigenemia and self-limiting CMV infection. We suppose that immunopathologic mechanisms could contribute to CMV-associated illness (22) in addition to direct cytopathic effects of the infection (20). Association of tumor necrosis factor and pulmonary immunopathologic features of active CMV infection was recently confirmed in a mouse model showing murine CMV reactivation after cecal ligation and puncture (9).
Deaths occurred later for patients with active CMV infection than for those without active CMV infection. This finding could be the consequence of CMV-associated disease, as has been suggested (17). Although our study was not designed to clarify the causality between active CMV infection and increased illness, we argue that active CMV infection increases illness and not vice versa. In the mouse model of CMV reactivation, the causality between sepsis, CMV reactivation, and pulmonary disease has already been shown (9).
Recently, reactivations of HSV and human herpesvirus 6 have been reported in critically ill patients (7,23). We demonstrated for the first time an association between active HSV and CMV infection (p = 0.004). HSV was isolated from bronchial aspirate in the absence of skin and mucosal lesions, whereas other herpesviruses, such as varicella-zoster virus, could not be isolated. The coincidence of HSV and CMV reactivation during the first 2 weeks of septic shock suggests a common trigger mechanism for herpesvirus reactivations. In future studies, more sensitive assays (e.g., PCR) may be used to analyze the incidence of other occult herpesvirus reactivations. Reactivation of polyoma BK virus, which commonly causes opportunistic infection after transplantation, was not detected by virus isolation. This finding leads to the hypothesis that the conditions that stimulate polyomavirus reactivation and those that stimulate CMV and HSV reactivation may differ. The absence of exogenous viral infection (e.g., adenovirus, respiratory syncytial virus, parainfluenzavirus) strengthens the suggestion that exogenous nosocomial viral infections are uncommon in patients in ICUs (24). Thus, monitoring for viral infections could focus on endogenous herpesvirus reactivations in patients with septic shock. Immunosuppression and proinflammatory cytokines, drugs, or combinations are presumed to be involved in herpesvirus reactivations; however, the exact mechanisms are still elusive for patients with septic shock (13,25).
After organ transplants, CMV-associated illness and death could be reduced by early antiviral therapy; however, delayed therapy has been less effective (2). Anecdotal reports show that critically ill patients with already established CMV organ disease may not benefit from antiviral therapy (3,4,20). The effect of preemptive antiviral therapy or antiviral prophylaxis has not been tested so far in patients with septic shock; however, in the mouse model, prophylactic treatment with ganciclovir prevented murine CMV reactivation and CMV-associated pulmonary fibrosis (9).
Despite the low patient number in this and previous studies, we suggest that CMV is a pathogen of emerging importance that can no longer be ignored for patients with septic shock. Thus, interventional studies aimed at preventing CMV-associated illness in patients with septic shock are needed.
Dr von Müller is a senior researcher in the Department of Virology, University Hospital Ulm, Ulm, Germany. He specializes in pediatrics, microbiology, and epidemiology of infectious diseases. His main research interest is clinical virology and the role of antiviral immunity.
Acknowledgments
We thank everyone involved in diagnosis and treatment of the critically ill patients for their excellent assistance.
This study was supported by Roche Diagnostics, Mannheim, Germany. The funding source was not involved in the study design, collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the manuscript for publication.
References
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. DOIPubMedGoogle Scholar
- Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 2675–2705.
- Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001;29:541–7. DOIPubMedGoogle Scholar
- Jaber S, Chanques G, Borry J, Souche B, Verdier R, Perrigault PF, Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005;127:233–41. DOIPubMedGoogle Scholar
- Stephan F, Meharzi D, Ricci S, Fajac A, Clergue F, Bernaudin JF. Evaluation by polymerase chain reaction of cytomegalovirus reactivation in intensive care patients under mechanical ventilation. Intensive Care Med. 1996;22:1244–9. DOIPubMedGoogle Scholar
- Desachy A, Ranger-Rogez S, Francois B, Venot C, Traccard I, Gastinne H, Reactivation of human herpesvirus type 6 in multiple organ failure syndrome. Clin Infect Dis. 2001;32:197–203. DOIPubMedGoogle Scholar
- Razonable RR, Fanning C, Brown RA, Espy MJ, Rivero A, Wilson J, Selective reactivation of human herpesvirus 6 variant a occurs in critically ill immunocompetent hosts. J Infect Dis. 2002;185:110–3. DOIPubMedGoogle Scholar
- Cook CH, Zhang Y, McGuinness BJ, Lahm MC, Sedmak DD, Ferguson RM. Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice. J Infect Dis. 2002;185:1395–400. DOIPubMedGoogle Scholar
- Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med. 2006;34:842–9.PubMedGoogle Scholar
- Docke WD, Prosch S, Fietze E, Kimel V, Zuckermann H, Klug C, Cytomegalovirus reactivation and tumour necrosis factor. Lancet. 1994;343:268–9. DOIPubMedGoogle Scholar
- Kutza AST, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998;26:1076–82. DOIPubMedGoogle Scholar
- Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–8. DOIPubMedGoogle Scholar
- Prosch S, Wendt CE, Reinke P, Priemer C, Oppert M, Kruger DH, A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology. 2000;272:357–65. DOIPubMedGoogle Scholar
- American College of Chest Physicians/Society of Critical Care Medicine consensus conference. definitions for sepsis and organ failure guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. DOIPubMedGoogle Scholar
- von Müller L, Hampl W, Hinz J, Meisel H, Reip A, Engelmann E, High variability between results of different in-house tests for cytomegalovirus (CMV) monitoring and a standardized quantitative plasma CMV PCR assay. J Clin Microbiol. 2002;40:2285–7. DOIPubMedGoogle Scholar
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–10. DOIPubMedGoogle Scholar
- Domart Y, Trouillet JL, Fagon JY, Chastre J, Brun-Vezinet F, Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery. Chest. 1990;97:18–22. DOIPubMedGoogle Scholar
- Cook CH, Yenchar JK, Kraner TO, Davies EA, Ferguson RM. Occult herpes family viruses may increase mortality in critically ill surgical patients. Am J Surg. 1998;176:357–60. DOIPubMedGoogle Scholar
- Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–6. DOIPubMedGoogle Scholar
- Papazian L, Thomas P, Bregeon F, Garbe L, Zandotti C, Saux P, Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology. 1998;88:935–44. DOIPubMedGoogle Scholar
- Brennan DC. Cytomegalovirus in renal transplantation. J Am Soc Nephrol. 2001;12:848–55.PubMedGoogle Scholar
- Barry SM, Johnson MA, Janossy G. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplant. 2000;26:591–7. DOIPubMedGoogle Scholar
- Cook CH, Martin LC, Yenchar JK, Lahm MC, McGuinness B, Davies EA, Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. 2003;31:1923–9. DOIPubMedGoogle Scholar
- Daubin C, Vincent S, Vabret A, du Cheyron D, Parienti JJ, Ramakers M, Nosocomial viral ventilator-associated pneumonia in the intensive care unit: a prospective cohort study. Intensive Care Med. 2005;31:1116–22. DOIPubMedGoogle Scholar
- Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–26. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 12, Number 10—October 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Thomas Mertens, Department of Virology, University Hospital Ulm Albert-Einstein-Allee 11, 89081 Ulm, Germany
Top