Volume 12, Number 6—June 2006
Research
Human Streptococcus suis Outbreak, Sichuan, China
Cite This Article
Citation for Media
Abstract
From mid-July to the end of August 2005, a total of 215 cases of human Streptococcus suis infections, 66 of which were laboratory confirmed, were reported in Sichuan, China. All infections occurred in backyard farmers who were directly exposed to infection during the slaughtering process of pigs that had died of unknown causes or been killed for food because they were ill. Sixty-one (28%) of the farmers had streptococcal toxic shock syndrome; 38 (62%) of them died. The other illnesses reported were sepsis (24%) and meningitis (48%) or both. All isolates tested positive for genes for tuf, species-specific 16S rRNA, cps2J, mrp, ef, and sly. A single strain of S. suis caused the outbreak, as shown by the identification of a single ribotype. The high death ratio was of concern; prohibiting backyard slaughtering ended the outbreak.
Streptococcus suis is a zoonotic microbe that can exist in pigs without causing illness but can occasionally cause disease. Serotype 2 is a dominant pathogenic serotype (1). Types 2 and 5 have been isolated from purulent lesions in the lungs and other extramammary sites in cattle, sheep, and goats (2). Infection may cause death in weaning piglets as well as growing pigs (3). The bacterium is isolated from an increasingly wide range of mammalian species, including horses, dogs, cats, and birds (4).
Sporadic cases of S. suis infection may occur in humans, the most common clinical manifestations included purulent meningitis, septicemia, arthritis, and endocarditis; some infections lead to sequelae such as deafness and ataxia (5–7). To date, ≈200 human cases have been reported in areas of intensive pig rearing (the Netherlands, Denmark) or areas where large quantities of pork are eaten (Hong Kong, Thailand, Vietnam) (8). Most reported cases of human S. suis infections were associated with contact with pigs or pork products (5). Human S. suis infections do not normally cause major outbreaks.
On July 11, 2005, a local hospital in Ziyang Prefecture of Sichuan Province reported a suspected case of hemorrhagic fever with renal syndrome. The patient was a 46-year-old male farmer with acute onset of high fever, lethargy, vomiting, and generalized purpura. The day before illness onset, the farmer slaughtered a pig that had died of an unknown cause. The farmer rapidly lapsed into coma. On further investigation, we identified 4 other patients with similar circumstances in the same hospital and more patients from other hospitals in the area. S. suis was isolated from blood cultures in some of these cases. We began an investigation of this outbreak to describe its epidemiologic, clinical, and microbiologic characteristics (9).
Epidemiologic Investigation
We reviewed medical records in all health care facilities in Ziyang Prefecture for patients admitted since June 10, 2005 (2 weeks before the onset of the first known case), with a diagnosis of septic shock or meningitis. On July 19, enhanced surveillance was introduced to include healthcare facilities in Ziyang and the 5 surrounding prefectures. We ordered all healthcare facilities to immediately report all new patients with clinical sepsis, meningitis, arthritis, or endocarditis and fever >37.3°C, who had epidemiologic risk factors (contact with sick pigs or any part, such as meat, skin, organs, or tissue, of pigs that had died of undetermined causes). Beginning on July 21, we made public announcements to encourage reporting. Public health workers interviewed patients, or surrogates for deceased patients, by using a questionnaire to collect demographic, clinical, and exposure information. Specimens (blood, cerebrospinal fluid [CSF], or postmortem tissue) were collected for laboratory investigation. We reviewed medical records to obtain supplementary clinical information. We placed all close contacts of case-patients, including family members and attending healthcare workers, under medical surveillance. We extended these surveillance procedures to all of Sichuan Province on July 25.
A probable case of S. suis infection was a compatible clinical illness (sepsis, meningitis, arthritis, or endocarditis), without laboratory evidence of infection by another organism, with history of contact with sick or dead domestic livestock (pigs, goats, or sheep) or another case-patient within 7 days before onset of symptoms. A confirmed case was defined as a compatible clinical illness regardless of exposure and the verification of S. suis isolated from a normally sterile site. We stopped the enhanced surveillance on August 18, two weeks after onset of the last case.
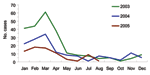
To assess the extent of underreporting, notification statistics on meningococcal meningitis were reviewed. Meningococcal meningitis is a statutorily notifiable disease in China. Because laboratory diagnosis was not routinely performed for all meningitis patients, the figures on suspected meningococcal cases could reflect nonmeningococcal meningitis caused by other bacteria, including S. suis. In this investigation, statistics in the 12 affected prefectures of Sichuan Province from January 2003 to August 2005 were reviewed.
Laboratory Investigation
Specimens of blood, CSF, or postmortem tissue from human patients and blood or postmortem tissue from affected pigs were injected onto sheep blood agar and infusion broth (REF 237500, Oxoid, Basingstoke, UK) in 0.5% CO2 at 37°C. S. suis strains were identified by examining growth colony shape, followed by biochemical reactions with Vitek2 compact and API 20 strep (bioMérieux, Inc., Beijing, China) according to the manufacturer's instructions.
Polymerase chain reaction (PCR) was used to characterize selected genes of S. suis serotype 2. PCR products were purified by using the QIAquick PCR purification kit (Qiagen, Valencia, CA, USA), according to the manufacturer's protocol, and sequenced with an ABI Prism 3700 DNA instrument (Applied BioSystems, Foster City, CA, USA). The following genes were sequenced, in accordance with methods published elsewhere: 1) genus-specific gene segments, tuf sequence, and 2) the species-specific gene coding for 16S rRNA of S. suis, the gene coding for the capsule of S. suis serotypes 2 (cps2J) and 1/2, the muramidase-released protein gene (mrp), suilysin (sly), and the extracellular factor gene (ef) (10–12). Sequence data were analyzed by using a basic local alignment search tool (BLAST) search performed against sequences published by the National Center for Biotechnology Information (Bethesda, MD, USA).
Automated PvuII and PstI ribotyping was performed by using the RiboPrinter microbial characterization system (DuPont China, Shenzhen, China), with bacterial isolates grown overnight on brain-heart infusion blood agar. Template preparation, restriction enzyme digestion, gel electrophoresis, and Southern hybridization with an Escherichia coli rrnB rRNA operon probe were carried out with the RiboPrinter system. Images were developed with a charge-coupled-device camera and analyzed by using the RiboPrinter's customized software.
Statistical Analysis
Percentage, proportion, and case-fatality ratios were calculated. The χ2 and rank sum tests were used to compare the case-fatality ratios and the median duration from exposure to onset between 2 groups, respectively, by using Stata version 8 (StataCorp LP, College Station, TX, USA). All probabilities were 2-tailed, and p<0.05 indicated significance.
The first case-patient was a 52-year-old male farmer from Yanjiang District of Ziyang Prefecture. On June 24, 2005, three days after he slaughtered and dressed a goat that had died of an unknown cause, fever (38.2°C), chills, abdominal pain, vomiting, generalized aching, and generalized purpura developed. Ten hours later, he died on the way to the hospital. The second case-patient was his neighbor who had similar symptoms on June 26 and died shortly after hospital admission. He had helped slaughter the goat with the first case-patient. Blood, tissue, and other specimens for culture were not available from these patients.
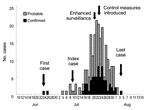
The Outbreak
Other cases of S. suis infection occurred in early July. The number of cases gradually increased, peaked during the second half of July, and dwindled rapidly thereafter (Figure 1). The decline coincided with new measures that prohibited domestic slaughter of sick pigs or pigs that died of any illness. These measures were implemented through provincial legislation and enforced with prosecution. The last case-patient had onset of illness on August 4. By August 18, two weeks after the date of onset of the last case, we had identified 215 cases (66 laboratory confirmed, 149 probable); 39 persons died in Sichuan Province. This finding compares to an expected number of suspected meningococcal disease cases of 10 per month reported through routine surveillance during the summer months (June to August) from 2003 to 2005 (Figure 2).
Cases of S. suis infection predominantly involved adult male farmers with recent exposure to sick pigs or carcasses of pigs that had died of unknown illnesses (Table 1). The most common methods of exposures were slaughtering a sick pig or preparing a pig carcass for meat, hides, and other pig products. Forty-eight percent of case-patients had wounds on their hands at the time of slaughter or carcass preparation. Illnesses that fit the case definition did not develop in close contacts of case-patients who did not participate in the slaughter, carcass preparation, or carcass disposal. None of the 417 healthcare workers who cared for case-patients became infected, and no similar clinical illness developed.
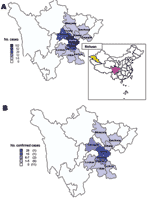
The residences of S. suis case-patients were widely distributed in 203 villages of 12 prefectures in Sichuan Province. In 194 villages, only 1 case was identified (Figure 3). In Ziyang, Yibin, and Chengdu, 2 case-patients in 1 village each were identified. The Ziyang cluster was composed of the first 2 case-patients in the outbreak. The Yibin cluster consisted of 1 farmer who slaughtered a sick pig and became ill with streptococcal toxic shock syndrome (STSS); another person who processed meat from the same pig became ill with meningitis and recovered. In Chengdu, 2 persons slaughtered a pig that had died of an unexplained illness; S. suis infection developed in both persons and they recovered. In 6 additional villages, >2 cases of S. suis infection occurred in patients who did not know each other and had no common exposure to a pig.
Clinical Patterns
All 215 affected persons were previously healthy adults. We observed 3 distinct clinical symptom settings (Table 2). First, 28% of patients had sepsis characterized by acute onset of fever, chills, headaches, dizziness, malaise, abdominal pain, and diarrhea. In some severe cases, patients became comatose. Second, 48% of patients had meningitis characterized by headache, stiff neck, and other signs of acute meningitis. Some meningitis patients also had disseminated intravascular coagulation and coma. Third, 28% of patients had STSS that has been described in other forms of streptococcal infections (13) and met the criteria established by Centers for Disease Control and Prevention (14). Patients with STSS had a 62% case-fatality ratio compared to 0.6% for other clinical forms of streptococcal infections. Fatal STSS cases progressed from onset to death in a median of 25 hours (range 8 hours to 10.5 days). Discharge records available on 85 survivors in all 3 clinical symptom settings showed a median interval from onset to recovery of 15 days (range 5–36 days).
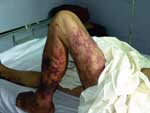
We determined exposure and onset times for 63 of the 66 laboratory-confirmed cases. Of the 215 cases, we could determine exposure time and onset times for 203. The median interval between exposure and onset was 2.2 days (range 3 hours to 14 days). The patients with STSS had a shorter incubation period and a higher frequency of gastrointestinal symptoms, coma, and petechiae and ecchymoses than did other patients (Table 3). Eight percent of non-STSS cases had petechiae or ecchymoses, and 9% had hypotension but did not fully fit the criteria for STSS. Typical skin manifestations are shown in Figure 4. Leukocytosis and thrombocytopenia were found in more than half of the patients (Table 4). Liver impairment was found in 74%, and renal function impairment was found in 19% of the patients who had been tested. CSF abnormalities compatible with purulent meningitis were found in 31 (40%) of 77 patients who had a lumbar puncture.
Postmortem examination of 4 STSS patients (2 confirmed and 2 probable infections) showed features of disseminated intravascular coagulation. Evidence of multiple organ damage was observed, primarily involving kidneys, adrenal glands, lungs, liver, pancreas, and heart. Histologic findings included microthrombosis (hyaline thrombus) in organ capillaries; necrosis of parenchymal cells; and congestion, exudate, and hemorrhage of interstitial vessels of kidneys, lungs, and other organs.
Microbiologic Investigations
We collected 348 specimens of blood (271), CSF (53), and tissue (24) from postmortem examination of 172 case-patients. We isolated S. suis from blood (36), CSF (27), and postmortem liver, spleen, and heart tissues (3). Fifty-five (83%) of the confirmed patients were diagnosed after July 23, 2005, representing 42% (55/130) (data not shown) of all tested samples, compared to that of 26% (11/42) (data not shown) before enhancement of surveillance.
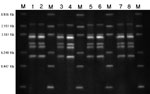
Isolates from 66 patients and 3 diseased pigs featured pure growth of tiny α-hemolytic colonies on sheep blood agar. The colony shape and biochemical reactions by Vitek2 compact and API-Strep were all compatible with that of S. suis. In the investigations after July 23, 2005, the results matched the suggested key indicators for the pathogen, including Voges-Proskauer negativity, hydrolysis of esculin, trehalose positivity, negativity for growth in 6.5% NaCl, and absence of β-hemolysis on sheep blood agar. PCR on all isolates showed gene coding for tuf, species-specific 16S rRNA of S. suis, genes coding for the capsule of S. suis serotypes 2 (cps2J) and 1/2, and mrp, sly, and ef. The PCR products of virulence genes of all 69 strains were sequenced. These were identical to the sequences published by the National Center for Biotechnology Information. On further investigation, only a single ribotype for either PvuII or PstI restriction was identified among the 25 S. suis type 2 isolates, including 22 from patients and 3 from diseased pigs (Figure 5), indicating a single clonal strain as the source of the infection (1).
We report an unprecedented outbreak of human S. suis serotype 2 infection involving 215 patients in Sichuan Province during the summer of 2005. This outbreak was characterized by the large number of patients involved, distinctive clinical manifestations, and the major challenges facing public health authorities in both surveillance and control.
This is the largest recorded outbreak of S. suis infection in humans. From late July to early August 1998, an outbreak of 25 cases with 14 deaths occurred in Jiangsu Province, China, from serotype 2 infection (15). The size of the outbreak in our study was probably related to a local farming practice. In Sichuan, a sizable swine population was found in small backyard farms; each family kept only a few animals. Farmers habitually slaughtered sick pigs for human consumption. Beginning mid-June 2005, a major outbreak of S. suis killed 647 pigs in almost the same areas as the outbreak in humans (16). The outbreak in swine peaked around July 20 with ≈4 dead pigs in each affected village. S. suis caused 98% of the deaths of these pigs in Sichuan during this period (17). All infected pigs came from backyard farms, usually with 1 sick pig in the herd. A pathogenic strain could have spread by distributing infected piglets to the backyard pig farms and then propagated among healthy pigs. The single ribotype identified in the investigation lends support to this theory.
The main risk factor for S. suis infection in the outbreak was direct involvement in slaughtering sick pigs and preparing carcasses of pigs that died of unknown causes. Unlike professionals in modern abattoirs, the local farmers did not wear protective gear or gloves. Normally 1–2 persons carried out the procedure, which involved bloodletting though a neck artery, manually inflating the carcasses, scalding the pigskin with ≈80°C water, and splitting and shaving the skin with large knives. Scalding and shaving were often performed together. The farmers then sliced the meat into smaller pieces before cooking for food. The complete process of slaughtering could take >1 hour. Our study demonstrated that all patients had been infected during direct contact with blood or tissues of sick or dead pigs. Often this may have occurred through direct exposure of skin wounds. Droplet exposure may also have occurred during slaughter or processing of carcasses, but we could not document this occurrence. The observed risk factors were consistent with those reported in other studies (5,6). No evidence of infection from eating cooked pork from these pigs was observed. The uncooked meat was shared with neighboring families, but these villagers normally do not eat raw meat or raw animal viscera. Person-to-person transmission was highly unlikely since we found no disease in family members, neighbors, or healthcare workers who had not been exposed to sick or dead pigs.
One missing link in the outbreak is the exact relationship between the dead goat in the early cluster of patients and the subsequent propagation of the S. suis infections. We could not confirm these 2 cases microbiologically. We speculate that S. suis caused these 2 early human infections because they occurred shortly after exposure to the dead goat, and because clinical manifestations in the 2 patients were similar to those of others in the outbreak. Human S. suis infection after exposure to sick goats has not been reported, despite isolation of the organism from these animals (2). In backyard farms where different animals are kept together, S. suis infection could have been transmitted between pigs and goats. Animal surveillance would help establish the role of animals other than pigs in carriage of the bacteria and the potential for causing human infections.
Clinically, 3 distinctive forms of human S. suis infection occurred, namely, STSS, sepsis, and meningitis. STSS has not been reported from S. suis infection, although it has been previously described in other streptococcal infections (13) and Staphylococcus aureus infections (18). Unusual STSS-like illnesses brought the outbreak to the attention of local health authorities. A dose effect may explain the relatively high proportion of STSS in this outbreak. While other explanations like comorbid conditions, e.g., asplenia, diabetes mellitus, alcoholism, and malignancy (19), have been reported, this was not the case for the outbreak in our study, which involved previously healthy adults.
Laboratory examination confirmed virulence factors in the S. suis isolates from this outbreak. They include mrp, sly, and ef, although their precise clinical role has not yet been shown. These isolates (mrp+, ef+, sly+) are related to European strains that are considered to be more virulent than North American strains (1). Genome analysis could determine if novel virulence genes were involved.
Finally, the high number of deaths due to STSS is a cause for concern. Prompt institution of effective responses to human S. suis outbreaks is a public health challenge, especially in rural China. Timely diagnosis is difficult. In our study, only 31% of the cases were laboratory confirmed. Suboptimal access to health services, personal delay in seeking treatment, underutilization of blood cultures in local hospitals, and self-administration of antimicrobial drugs may explain the relatively low proportion of culture-positive human cases, especially during the early phase of the outbreak. Many patients died without having sought treatment from any health facility. As for the investigation process, the existing surveillance system that covers meningitis is not robust enough to alert public health personnel of the impending threat of S. suis. Control measures included prohibiting by law of slaughtering, eating, selling, and transporting deceased or sick pigs. Subsidies were offered to families to support hygienic handling of deceased or sick pigs and to patients for medical care. Village heads were held accountable for illegal slaughtering in their village. These measures were supplemented with disinfection of affected backyard farms. Public education campaigns were staged to increase awareness of how to prevent and control human S. suis infections. In the long run, the prevention and control of swine infection should form the more strategic component of the public health program. Surveillance systems should be established to alert farmers and the general public if an infection outbreak in pigs is recognized (19).
Dr Yu is a medical epidemiologist in the Office for Disease Control and Emergency Response, Chinese Center for Disease Control and Prevention. His research interests include surveillance, prevention, and control of emerging infections, including severe acute respiratory syndrome, avian influenza, and S. suis.
Acknowledgments
We thank the Sichuan Health Bureau, the government of Ziyang, the Center for Disease Control and Prevention of Chengdu, and Ziyang, Neijiang, Suining, Mianyang, Zigong, Luzhou, Yibing, Deyang, Leshan, Nanchong, and Guangan Prefectures for assisting in coordinating field investigations and providing logistics support. The Ministry of Health in China has generously facilitated this study. We thank Lee Chin-Kei and Robert E. Fontaine for helping us prepare the article.
The Chinese Center for Disease Control and Prevention supported this study both technically and administratively.
References
- Berthelot-Herault F, Gottschalk M, Morvan H, Kobisch M. Dilemma of virulence of Streptococcus suis: Canadian isolate 89-1591 characterized as a virulent strain using a standardized experimental model in pigs. Can J Vet Res. 2005;69:236–40.PubMedGoogle Scholar
- Acha PN, Szyfres B. Zoonoses and communicable diseases common to man and animals. Vol 1. Bacterioses and mycoses. 3rd ed. Washington: Pan American Health Organization; 2003. p. 257–65.
- Higgins R, Gottschalk M. Streptococcal diseases. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of swine. Ames (IA):Iowa State University Press; 1999. p. 563–78.
- Krauss H, Weber A, Appel M, eds. Zoonoses—infectious diseases transmissible from animals to humans, 3rd ed. Washington: ASM Press; 2003. p. 239-40.
- Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. QJM. 1995;88:39–47.PubMedGoogle Scholar
- Suankratay C, Intalapaporn P, Nunthapisud P, Anmyingmongkol K, Wilde H. Streptococcus suis meningitis in Thailand. Southeast Asian J Trop Med Public Health. 2004;35:868–76.PubMedGoogle Scholar
- Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10:131–7. DOIPubMedGoogle Scholar
- Strangmann E, Froleke H, Kohse KP. Septic shock caused by Streptococcus suis: case report and investigation of a risk group. Int J Hyg Environ Health. 2002;205:385–92. DOIPubMedGoogle Scholar
- World Health Organization. Outbreak associated with Streptococcus suis in pigs, China. Wkly Epidemiol Rec. 2005;80:269–76.PubMedGoogle Scholar
- Tarradas C, Arenas A, Maldonado A, Luque I, Miranda A, Perea A. Identification of Streptococcus suis isolated from swine: proposal for biochemical parameters. J Clin Microbiol. 1994;32:578–80.PubMedGoogle Scholar
- Marois C, Bougeard S, Gottschalk M, Kobisch M. Multiplex PCR assay for detection of Streptococcus suis species and serotypes 2 and 1/2 in tonsils of live and dead pigs. J Clin Microbiol. 2004;47:3169–75. DOIPubMedGoogle Scholar
- Picard FJ, Ke D, Boudreau DK, Boissinot M, Huletsky A, Richard D, Use of tuf sequences for genus-specific PCR detection and phylogenetic analysis of 28 streptococcal species. J Clin Microbiol. 2004;42:3686–95. DOIPubMedGoogle Scholar
- Hashikawa S, Iinuma Y, Furushita M, Ohkura T, Nada T, Torii K, Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J Clin Microbiol. 2004;42:186–92. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Streptococcal toxic-shock syndrome (STSS), 1996 case definition [monograph on the Internet]. 2004 Apr [cited 2005 Aug 20]. Available from http://www.cdc.gov/epo/dphsi/casedef/streptococcalcurrent.htm
- Hu X, Zhu F, Wang H, Chen S, Wang G, Sun J, Studies on human streptococcal infectious syndrome caused by infected pigs [article in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi. 2000;34:150–2.PubMedGoogle Scholar
- Office International des Epizooties. The People's Republic of China notifies officially to the OIE an outbreak of Streptococcus suis in pigs, including laboratory investigation research in animals [monograph on the Internet]. 2005 Aug [cited 2005 Aug 20]. Available from http://www.oie.int/eng/press/en050805b.htm
- Ministry of Health. People's Republic of China. Estimation report of Streptococcus suis infection in Sichuan province [monograph on the Internet]. 2005 Aug [cited 2006 Feb 5]. Available from http://www.moh.gov.cn/public/open.aspx?n_id=10317&seq=0
- Shands KN, Schmid GP, Dan BB, Blum D, Guidotti RJ, Hargrett NT, Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303:1436–42. DOIPubMedGoogle Scholar
- Huang YT, Teng LJ, Ho SW, Hsueh PR. Streptococcus suis infection. J Microbiol Immunol Infect. 2005;38:306–13.PubMedGoogle Scholar
Figures
Tables
Cite This Article1These authors contributed equally to this article.
2Members of the Chinese Center for Disease Control and Prevention Streptococcus suis study group are Wenjun Zhong, Ling Meng, Yongjun Gao, Huamao Du, Changyu Ye, Zhigang Cui, Shouyin Zhang, and Dong Jin. Members of the Sichuan Center for Disease Control and Prevention Streptococcus suis study group are Li Liu, Heng Yuan, Bin Ouyang, Qiang Lv, Yan Huang, Ting Huang, Xingyu Zhou, Liao Feng, and Qidi Pang.
Table of Contents – Volume 12, Number 6—June 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Weizhong Yang, Office for Disease Control and Emergency Response, China CDC, 27 Nanwei Rd, Beijing, 100050, People’s Republic of China
Top