Volume 13, Number 12—December 2007
Perspective
Need for Improved Methods to Collect and Present Spatial Epidemiologic Data for Vectorborne Diseases
Cite This Article
Citation for Media
Abstract
Improved methods for collection and presentation of spatial epidemiologic data are needed for vectorborne diseases in the United States. Lack of reliable data for probable pathogen exposure site has emerged as a major obstacle to the development of predictive spatial risk models. Although plague case investigations can serve as a model for how to ideally generate needed information, this comprehensive approach is cost-prohibitive for more common and less severe diseases. New methods are urgently needed to determine probable pathogen exposure sites that will yield reliable results while taking into account economic and time constraints of the public health system and attending physicians. Recent data demonstrate the need for a change from use of the county spatial unit for presentation of incidence of vectorborne diseases to more precise ZIP code or census tract scales. Such fine-scale spatial risk patterns can be communicated to the public and medical community through Web-mapping approaches.
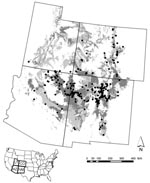
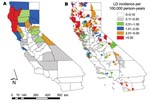
Risk for human exposure to arthropod vectors and their associated pathogens (e.g., the tickborne Lyme disease spirochete Borrelia burgdorferi, fleaborne plague bacterium Yersinia pestis, and mosquitoborne West Nile virus [WNV]) is spatially highly heterogeneous in the United States (1–16). This concept can be exemplified by the spatial distributions of plague cases and areas with high projected plague risk in Arizona, New Mexico, Utah, and Colorado (Figure 1) and incidence of endemic Lyme disease in California (Figure 2) (8,9). Such heterogeneity in spatial risk patterns results in part from variability in environmental suitability for the vectors, especially with regard to climate factors and habitat type, and abundance of vertebrate hosts or pathogen reservoirs (17–21). Three examples can illustrate this point. First, exposure to Ixodes pacificus nymphs, which serve as primary vectors of B. burgdorferi in California, is largely restricted to dense woodlands with a ground cover dominated by leaf litter and lacking emergent vegetation (22,23). Moreover, density of nymphs and B. burgdorferi–infected nymphs differs between different woodland types; oak woodlands show a greater risk for exposure to the Lyme disease agent than redwood habitats (6,7). These differences represent crucial knowledge in assessment of probable pathogen exposure sites for Lyme disease cases in California.
Second, spatial patterns of distribution and abundance of the mosquito Culex tarsalis, which is considered the primary vector to humans of WNV in the western United States, are related to both climatic conditions and suitable mosquito larval habitats (13,14,24–28). In Colorado, which had a WNV disease outbreak with 2,947 reported human cases in 2003, the spatial pattern of abundance of Cx. tarsalis is highly heterogeneous. The mosquito occurs commonly only at lower elevations <1,800 m (24,25), and its presence in the semiarid plains landscape characteristic of eastern Colorado is strongly correlated with availability of water sources (natural or resulting from irrigation) for the immature aquatic mosquito life stages. Assessments of probable WNV exposure sites in Colorado are complicated by inadequate knowledge of the fine-scale spatial distributions of key Culex spp. WNV vectors (Cx. pipiens, Cx. tarsalis) and the fact that people commonly are bitten by other mosquitoes in areas where these vectors and WNV are absent (e.g., the high mountains in central Colorado).
Third, human plague cases in the southwestern United States are closely associated with ecotonal piñon-juniper habitat and elevation (9). The etiologic agent of plague is transmitted primarily by flea bite, and human cases are typically associated with epizootic activity, which most commonly occurs in clearly defined habitat types and under climatic conditions favoring build-up of dense rodent and flea populations (17,18,29,30). Exhaustive plague case investigations by state health agencies or the Centers for Disease Control and Prevention (CDC) ensure reliable assessments of probable exposure sites for Y. pestis in the United States.
Over the past decade, advances in geographic information system technology have facilitated the development of predictive spatial models for risk for exposure to key vectors and pathogens in the United States (1,3,5,7–12,16). However, lack of reliable data for probable pathogen exposure sites has emerged as a major obstacle to the development of spatial epidemiologic and ecoepidemiologic models. In the United States, comprehensive case investigations by teams that include epidemiologists and vector ecologists and the determination of probable pathogen exposure sites are routinely conducted only for plague. Although the plague case investigation can serve as a model for how to ideally generate needed information for probable pathogen exposure sites, this exhaustive approach is cost-prohibitive for more common and less severe diseases such as Lyme disease and WNV disease. Unless the public health system is willing to invest funds needed to conduct comprehensive case investigations for a given vectorborne disease, determinations of probable pathogen exposure sites will remain the responsibility of the attending physician. Physicians may not be willing to spend the time required to obtain extensive patient travel histories to determine probable pathogen exposure site, and their lack of training in vector ecology impedes their ability to collect relevant information.
To solve this problem, new methods are needed to determine probable pathogen exposure site that yield reliable results while taking into account economic and time constraints of the public health system and attending physicians. These methods could, for example, include sets of standardized questions developed by CDC and tailored to a given vectorborne disease. A critical minimal need includes a basic assessment of whether pathogen exposure likely occurred in 1) the peridomestic environment, 2) outside the peridomestic environment but within the county of residence, or 3) outside the county of residence. The role of this issue for spatial epidemiologic modeling was demonstrated by our recent study of Lyme disease in California where reexamination of Lyme disease case files from 1993 through 2005 showed that 27% of the 1,325 case-patients had likely been exposed to the pathogen outside the county of residence (8). Other possible approaches include the point-radius method for georeferencing of probable pathogen exposure sites on the basis of patient activity space patterns (31). Research is needed to determine the value and feasibility of implementing these or other methods into routine public health activities.
CDC and individual state health agencies routinely use county as the spatial unit for calculating and presenting incidence of vectorborne disease. The main problem with using county-based incidences for vectorborne diseases is that incidences calculated at this relatively crude spatial scale obscure fine-scale risk patterns commonly occurring within a county. This is especially problematic in the western United States, where many counties cover extensive areas (Figure 3) and encompass considerable ecologic and climatic variability. It was therefore not surprising that Eisen et al. (8) found that calculation and presentation of incidence of endemic Lyme disease in California at the county spatial unit, relative to the 5-digit ZIP code spatial unit, served to obscure small, isolated high-risk areas in the southern part of the state and the spatial variability of risk within high-risk counties (Figure 2).
Knowledge of local areas and habitats representing risk for vector exposure can be a major component in a diagnosis of probable Lyme disease or plague because infected persons may be unaware of receiving a tick or flea bite (32–34). Such knowledge is crucial in areas of the United States where the disease in question occurs but is rare. For example, in the absence of a documented tick bite and without knowledge that there are local areas with risk for exposure to the Lyme disease agent, a physician may be unlikely to consider Lyme disease as a possible diagnosis unless the patient has visited some other area the physician perceives to pose risk for exposure to the Lyme disease agent. Recognizing heterogeneity in spatial risk patterns for plague is similarly critical because it will aid local public health workers in targeting education of healthcare providers and the public to areas with a high risk for exposure to the plague agent (9,35). Prevention and treatment guidelines are well established for plague, but outcome of infection is improved by early diagnosis followed by appropriate treatment with antimicrobial drugs (36).
The 2 primary options in a shift away from using the county spatial unit for vectorborne disease incidence calculations are 1) ZIP code/ZIP code tabulation area and 2) census tract. There are pros and cons for each option. The 5-digit ZIP code unit is convenient because information regarding ZIP code of home address is readily collected during a visit to a physician, and the public is well aware of their ZIP code of residence and therefore can make ready use of information in map formats for ZIP code–based risk patterns. Conversely, a recent publication (37) raised concerns regarding increasing use of ZIP codes/ZIP code tabulation areas in spatial analyses of epidemiologic data because of their lack of standardization and dynamic spatial structure.
The more permanent census tract spatial unit, which tends to be smaller than the 5-digit ZIP code unit in population centers but can be larger than the ZIP code unit in sparsely populated areas, is attractive because it has a more uniform population base (typically 1,500–8,000 persons) than the ZIP code unit and therefore is less prone to the problem of overestimation of disease incidence on the basis of a few cases among a low population base. As demonstrated for WNV disease in Colorado by the Colorado Department of Public Health and Environment, some state level agencies have already adopted the practice of using the census tract unit to present spatial patterns of vectorborne diseases (www.cdphe.state.co.us/dc/zoonosis/wnv). Research is needed to evaluate the relative benefits of using the ZIP code compared with the census tract unit for calculation and presentation of spatial patterns of different vectorborne diseases.
Finally, advances in geographic information system technology and the ever-increasing use of the Internet as a primary knowledge resource provide tremendous possibilities for disseminating information regarding spatially explicit risk for exposure to vectorborne pathogens. Using a Web-mapping approach, one could easily convert static maps for plague and Lyme disease (Figures 1, 2) into a Web-based information delivery system in which selecting a county of interest provides a closeup view of the county, showing risk patterns for labeled ZIP codes and the location of major roads, population centers, and heavily used recreation areas.
New methods of determining probable pathogen exposure site that yield reliable results while taking into account economical and time constraints of the public health system are urgently needed to improve capability for developing predictive spatial risk models for vectorborne diseases in the United States. Recent data also demonstrate the need for a change from use of the crude county spatial unit for presentation of incidence of vectorborne diseases to finer ZIP code or census tract scales. Communication of such fine-scale spatial risk patterns to the public and medical community can be achieved through Web-mapping approaches.
Dr Lars Eisen is an assistant professor at Colorado State University in Fort Collins. His research interests include the ecology, epidemiology, and prevention of vectorborne disease.
Dr Rebecca J. Eisen is a service fellow in the Bacterial Diseases Branch, Division of Vector-Borne Infectious Diseases, CDC, Fort Collins, Colorado. Her research interests include the ecology, epidemiology, and prevention of vectorborne disease.
Acknowledgment
We thank A.T. Peterson for helpful comments.
References
- Brownstein JS, Rosen H, Purdy D, Miller JR, Merlino M, Mostashari F, Spatial analysis of West Nile virus: rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne Zoonotic Dis. 2002;2:157–64. DOIPubMedGoogle Scholar
- Brownstein JS, Holford TR, Fish D. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ Health Perspect. 2003;111:1152–7.PubMedGoogle Scholar
- Bunnell JE, Price SD, Das A, Shields TM, Glass GE. Geographic information systems and spatial analysis of adult Ixodes scapularis (Acari: Ixodidae) in the middle Atlantic region of the USA. J Med Entomol. 2003;40:570–6. DOIPubMedGoogle Scholar
- Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol. 1998;35:629–38.PubMedGoogle Scholar
- Diuk-Wasser MA, Brown HE, Andreadis TG, Fish D. Modeling the spatial distribution of mosquito vectors for West Nile virus in Connecticut, USA. Vector Borne Zoonotic Dis. 2006;6:283–95. DOIPubMedGoogle Scholar
- Eisen RJ, Eisen L, Castro MB, Lane RS. Environmentally related variability in risk of exposure to Lyme disease spirochetes in northern California: effect of climatic conditions and habitat type. Environ Entomol. 2003;32:1010–8. DOIGoogle Scholar
- Eisen RJ, Eisen L, Lane RS. Predicting density of Ixodes pacificus nymphs in dense woodlands in Mendocino County, California, based on geographic information systems and remote sensing versus field-derived data. Am J Trop Med Hyg. 2006;74:632–40.PubMedGoogle Scholar
- Eisen RJ, Lane RS, Fritz CL, Eisen L. Spatial patterns of Lyme disease risk in California based on disease incidence data and modeling of vector-tick exposure. Am J Trop Med Hyg. 2006;75:669–76.PubMedGoogle Scholar
- Eisen RJ, Enscore RE, Biggerstaff BJ, Reynolds PJ, Ettestad P, Brown T, Human plague in the southwestern United States, 1957–2004: spatial models of elevated risk of human exposure to Yersinia pestis. J Med Entomol. 2007;44:530–7. DOIPubMedGoogle Scholar
- Guerra M, Walker E, Jones C, Paskewitz S, Cortinas MR, Stancil A, Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the north-central United States. Emerg Infect Dis. 2002;8:289–97. DOIPubMedGoogle Scholar
- Kitron U, Kazmierczak JJ. Spatial analysis of the distribution of Lyme disease in Wisconsin. Am J Epidemiol. 1997;145:558–66.PubMedGoogle Scholar
- Nicholson MC, Mather TN. Methods for evaluating Lyme disease risks using geographic information systems and geospatial analysis. J Med Entomol. 1996;33:711–20.PubMedGoogle Scholar
- Reeves WC, Hammon WM, Longshore WA Jr, McClure H, Geib AF. Epidemiology of the arthropod-borne virus encephalitides in Kern County, California, 1943–1952. University of California Publications in Public Health. 1962;4:1–257.
- Reisen WK, Lothrop HD, Presser SB, Milby MM, Hardy JL, Wargo MJ, Landscape ecology of arboviruses in southern California: temporal and spatial patterns of vector and virus activity in Coachella valley, 1990–1992. J Med Entomol. 1995;32:255–66.PubMedGoogle Scholar
- Ruiz MO, Tedesco C, McTighe TJ, Austin C, Kitron U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr. 2004;3:8. DOIPubMedGoogle Scholar
- Yabsley MJ, Wimberly MC, Stallknecht DE, Little SE, Davidson WR. Spatial analysis of the distribution of Ehrlichia chaffeensis, causative agent of human monocytotropic ehrlichiosis, across a multi-state region. Am J Trop Med Hyg. 2005;72:840–50.PubMedGoogle Scholar
- Gage KL, Ostfeld RS, Olson JG. Nonviral vector-borne zoonoses associated with mammals in the United States. J Mammal. 1995;76:695–715. DOIGoogle Scholar
- Gage KL, Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol. 2005;50:505–28. DOIPubMedGoogle Scholar
- Kitron U. Landscape ecology and epidemiology of vector-borne diseases: tools for spatial analysis. J Med Entomol. 1998;35:435–45.PubMedGoogle Scholar
- Peterson AT. Ecologic niche modeling and spatial patterns of disease transmission. Emerg Infect Dis. 2006;12:1822–6.PubMedGoogle Scholar
- Wilson ML. Distribution and abundance of Ixodes scapularis (Acari: Ixodidae) in North America: ecological processes and spatial analysis. J Med Entomol. 1998;35:446–57.PubMedGoogle Scholar
- Tälleklint-Eisen L, Lane RS. Spatial and temporal variation in the density of Ixodes pacificus (Acari: Ixodidae) nymphs. Environ Entomol. 2000;29:272–80. DOIGoogle Scholar
- Clover JR, Lane RS. Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of Lyme disease in California. Am J Trop Med Hyg. 1995;53:237–40.PubMedGoogle Scholar
- Baker M. The altitudinal distribution of mosquito larvae in the Colorado Front Range. Trans Am Entomol Soc. 1961;87:231–46.
- Bolling BG, Moore CG, Anderson SL, Blair CD, Beaty BJ. Entomological studies along the Colorado Front Range during a period of intense West Nile virus activity. J Am Mosq Control Assoc. 2007;23:37–46. DOIPubMedGoogle Scholar
- Reeves WC, Hardy JL, Reisen WK, Milby MM. Potential effect of global warming on mosquito-borne arboviruses. J Med Entomol. 1994;31:323–32.PubMedGoogle Scholar
- Wegbreit J, Reisen WK. Relationships among weather, mosquito abundance, and encephalitis virus activity in California: Kern County 1990–98. J Am Mosq Control Assoc. 2000;16:22–7.PubMedGoogle Scholar
- Zou L, Miller SN, Schmidtmann ET. Mosquito larval habitat mapping using remote sensing and GIS: implications of coalbed methane development and West Nile virus. J Med Entomol. 2006;43:1034–41. DOIPubMedGoogle Scholar
- Barnes AM. Surveillance and control of bubonic plague in the United States. Symposium of the Zoological Society of London. 1982;50:237–70.
- Eisen RJ, Bearden SW, Wilder AP, Montenieri JA, Antolin MF, Gage KL. Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proc Natl Acad Sci U S A. 2006;103:15380–5. DOIPubMedGoogle Scholar
- Wieczorek J, Guo Q, Hijmans R. The point-radius method for georeferencing locality descriptions and calculating associated uncertainty. Int J Geogr Inf Sci. 2004;18:745–67. DOIGoogle Scholar
- Walker DH. Tick-transmitted infectious diseases in the United States. Annu Rev Public Health. 1998;19:237–69. DOIPubMedGoogle Scholar
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424–30. DOIPubMedGoogle Scholar
- Mann JM, Martone WJ, Boyce JM, Kaufmann AF, Barnes AM, Weber NS. Endemic human plague in New Mexico: risk factors associated with infection. J Infect Dis. 1979;140:397–401.PubMedGoogle Scholar
- Gage KL. Plague surveillance. In: Dennis DT, Gage KL, Grantz N, Poland PD, Tikhomirov E, editors. Plague manual: epidemiology, distribution, surveillance, and control. Geneva: World Health Organization; 1999. p. 135–65.
- Levy CE, Gage KL. Plague in the United States, 1995–1997. Infect Med. 1999;16:54–64.
- Grubesic TH, Matisziw TC. On the use of ZIP codes and ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. Int J Health Geogr. 2006;5:58–72. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 13, Number 12—December 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Lars Eisen, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, CO 80523, USA;
Top