Volume 13, Number 2—February 2007
Dispatch
Mosquitoborne Infections after Hurricane Jeanne, Haiti, 2004
Cite This Article
Citation for Media
Abstract
After Hurricane Jeanne in September 2004, surveillance for mosquitoborne diseases in Gonaïves, Haiti, identified 3 patients with malaria, 2 with acute dengue infections, and 2 with acute West Nile virus infections among 116 febrile patients. These are the first reported human West Nile virus infections on the island of Hispaniola.
Hurricane Jeanne caused large-scale devastation in Gonaïves, Haiti, on September 18, 2004. The US Department of Health and Human Services assisted the Haitian Ministry of Health by conducting a rapid field assessment of health-related issues. Among the actions recommended by the team was immediate epidemiologic assistance from the Centers for Disease Control and Prevention (CDC) to reinforce and expand epidemiologic surveillance to identify as early as possible any emerging epidemic or community health problems. Concern was raised that the combination of flooding, loss of shelter, and destruction of infrastructure would result in an outbreak of mosquitoborne diseases. We conducted surveillance to assess the extent of mosquitoborne diseases and monitor for outbreaks of these diseases.
We established laboratory-based fever surveillance at the 3 clinics providing healthcare in Gonaïves after the passage of Hurricane Jeanne. Febrile patients (core temperature ≥38.5°C when first assessed) were asked to provide blood for a serum sample and thick and thin malaria smears. The attending physician recorded each patient’s medical history, conducted a physical examination, and reported the discharge diagnosis and the therapy that was provided. We asked patients to return in 2 weeks so that a convalescent-phase serum sample could be collected.
Malaria smears were stained and read by using standard methods (1) at CDC. To diagnose dengue, we used nested PCR and the TaqMan assay to detect dengue viral RNA in serum samples obtained ≤5 days after onset of symptoms (2,3). In addition, we used an immunoglobulin M (IgM) antibody-capture (MAC)–ELISA to detect anti-dengue IgM antibodies in all serum specimens (4) at CDC. A result was considered positive when optical density, after comparison to negative serum and control antigen, was >0.20. All serum specimens were also tested for the presence of IgG antibodies to determine previous exposure to flaviviruses by using an IgG ELISA. In paired samples, a full titration of 4-fold dilutions of serum was used. The endpoint titration of IgG was determined to assess seroconversion (5). Each plate was compared with a negative control serum specimen. Because of cross-reactivity between anti-flavivirus antibodies, we used a microsphere-based immunoassay (MIA) with a quadratic discrimination analysis (6) and a plaque reduction neutralization test (PRNT) to distinguish between infecting flaviviruses. For the PRNT, serial dilutions of heat-inactivated serum were incubated with defined amounts of West Nile, Saint Louis encephalitis, and dengue viruses 1–4 for 2 hours at room temperature. The nonneutralized viral fraction was subsequently adsorbed onto a monolayer of Vero cells for 1 hour. The resultant plaques were counted and compared with results for the control virus with no serum. The endpoint of the titration was the highest dilution of serum that reduced the number of plaques 90% compared with the control results.
From November 15 through December 22, 2004, 116 acutely febrile patients were identified and included in our surveillance. Ages ranged from 4 months to 71 years (median 4 years); 52% were female. All patients lived in Gonaïves. Seventy-one patients (61%) appeared for treatment with a chief complaint of fever with cough, 35 (30%) had fever with no apparent source, 6 (5%) reported fever with diarrhea, and 4 (3%) reported fever with rash. Patients sought treatment a median of 3 days after the onset of fever (range 0–28 days). In addition to fever, the most commonly reported symptoms were cough (77, 66%), abdominal pain (57, 49%), and headache (56, 48%). Thirty-nine patients (34%) had at least 1 clinical sign of dehydration; 16 patients (14%) were hypotensive on physical examination. No patients were jaundiced or had spontaneous hemorrhage. The most common clinical diagnoses were upper respiratory infection (35, 30%), malaria (34, 29%), pneumonia (21, 18%), and typhoid fever (13, 11%). No cases of dengue fever were suspected. Fifty-eight patients (50%) were treated with oral antimicrobial drugs; 13 patients (11%) were prescribed chloroquine, and 2 patients (2%) received an antihelminthic drug. All cases of suspected malaria were diagnosed by patients’ clinical symptoms. Suspected tuberculosis was confirmed in 1 patient by a positive sputum smear. None of 116 patients was admitted to a hospital.
Of the 116 thick and thin smears, 3 (3%) samples showed a high level of parasitemia with Plasmodium falciparum. The 3 corresponding patients had fever with no apparent source. Malaria was suspected in 2 of the patients by their clinical symptoms; the third patient was thought to have typhoid and was treated with trimethoprim-sulfamethoxazole.
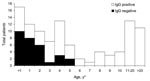
Two patients (2%) had acute, secondary dengue infections that were confirmed as positive by both IgM and IgG serologic tests. Both patients had a chief report of fever with no source, but malaria was suspected by the attending physician, and 1 patient was treated with chloroquine. We were not able identify dengue viral particles in the serum specimens of these patients. However, 79 patients (68%) were positive for anti-dengue IgG, which suggests a high level of flavivirus transmission in this area in the recent past (Figure).
Two patients (2%) had MIA results consistent with acute West Nile virus infection. The results were confirmed by PRNT (Table). Both patients were febrile in the clinic; 1 was a 13-year-old boy and the other was an infant girl <1 year of age. In addition to fever, the 13-year-old reported headache and abdominal pain, while cough was reported in the infant. Acute malaria was clinically diagnosed in both patients. The older child received chloroquine, while the younger child received only acetaminophen for fever control.
This surveillance program was established to assess the incidence of vectorborne diseases in the wake of Hurricane Jeanne. A total of 116 acutely febrile patients had blood drawn to determine whether a mosquitoborne disease was the etiologic agent of fever. An outbreak of mosquitoborne disease was not detected during the period of surveillance. Our data are consistent with previously published reports, which indicate that the incidence of arboviral infections rarely increases after water-related disasters (e.g., floods, hurricanes) (7−9). However, malaria outbreaks are common in such settings (9−11).
Despite the absence of an outbreak, our surveillance did identify the ongoing transmission of 3 mosquitoborne pathogens. Specifically, we diagnosed 3 cases of acute malaria, 2 cases of acute dengue, and 2 cases of acute West Nile virus infection. We also detected a high seroprevalence of dengue infections in children, which suggests substantial local dengue transmission in the Gonaïves area in the recent past.
The high seroprevalence of dengue and the low smear-positive rate of malaria from our surveillance were consistent with previously reported studies in this region of Haiti (12,13). The identification of 2 patients with positive West Nile virus results in Haiti is new. The only other human West Nile virus infections identified in the Caribbean Basin were 1 case reported in a Cayman Islands resident in 2001 (14) and 2 cases reported in Cuba, 1 in 2003 and the other in 2004 (15). This finding is not unexpected, however, because Komar et al. have identified West Nile virus in bird species native to the Dominican Republic, located to the east of Haiti on the island of Hispaniola.
The fact that the rate of West Nile virus infection was equal to the rate of acute dengue infection among our participants is of concern. Moreover, because both viruses can cause a nonlocalizing fever, the potential for confusion with malaria exists. Differentiating the cause of acute nonlocalizing febrile illnesses by examining malaria smears before initiating therapy, especially in an area with a history of low smear positivity, is therefore important.
Dr Beatty was previously the epidemiology and prevention activity leader at the Dengue Branch of CDC in San Juan, Puerto Rico. He joined the International Vaccine Institute in Seoul, Korea, in 2006. Dr Beatty’s research interests include arboviral and enteric infectious diseases.
Acknowledgment
We thank Eric Mintz for his technical support during the project. We also thank the medical and support staff of Haitian Ministry of Health, the Haiti-Global Aids Program of the Centers for Disease Control and Prevention, Médecins du Monde, Médecins Sans Frontières, and the International Federation of Red Cross and Red Crescent Societies for their assistance with this project.
References
- Division of Parasitic Diseases. Centers for Disease Control and Prevention [homepage on the internet]. Atlanta (GA): Diagnostic procedures for blood specimens [modified May 27, 2003]. In: Laboratory Identification of Parasites of Public Health Concern [cited 27 Dec 2005]. Available from www.dpd.cdc.gov/dpdx/HTML/DiagnosticProcedures.htm
- Lanciotti RS, Calisher CH, Gubler DJ, Chang JG, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples using reverse transcriptase-polymerase chain reaction.J Clin Microbiol. 1992;30:545–51.PubMedGoogle Scholar
- Chien LJ, Liao TL, Shu PY, Huang JH, Gubler DJ, Chang GJ. An objective approach for the development of real-time reverse transcriptase-PCR assays to detect and serotype dengue viruses.J Clin Microbiol. 2006;44:1295–304. DOIPubMedGoogle Scholar
- Burke DS, Nisalak A, Ussery MA. Antibody capture immunoassay detection of Japanese encephalitis virus immunoglobulin M and G antibodies in cerebrospinal fluid.J Clin Microbiol. 1982;16:1034–42.PubMedGoogle Scholar
- Miagostovich MP, Nogueira RMR, dos Santos FB, Schatzmayr HG, Araujo ESM, Vorndam V. Evaluation of an IgG enzyme–linked immunosorbent assay for dengue diagnosis.J Clin Virol. 1999;14:183–9. DOIPubMedGoogle Scholar
- Johnson AJ, Noga AJ, Kosoy O, Lanciotti RS, Johnson AA, Biggerstaff BJ. Duplex microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin m antibodies.Clin Diagn Lab Immunol. 2005;12:566–74.PubMedGoogle Scholar
- Rigau-Perez JG, Ayala-Lopez A, Garcia-Rivera EJ, Hudson SM, Vorndam V, Reiter P, The reappearance of dengue-3 and a subsequent dengue-4 and dengue-1 epidemic in Puerto Rico in 1998.Am J Trop Med Hyg. 2002;67:355–62.PubMedGoogle Scholar
- Nasci RS, Moore CG. Vector-borne disease surveillance and natural disasters.Emerg Infect Dis. 1998;4:333–4.PubMedGoogle Scholar
- Pan American Health Organization, Situación de las enfermedades infecciosas de mayor riesgo epidemiológico en el período post-Mitch países de Centroamérica, 1998. OPS/HCP/HCT/134/98.
- Kondo H, Seo N, Yasuda T, Hasizume M, Koido Y, Ninomiya N, Post-flood—infectious diseases in Mozambique.Prehosp Disaster Med. 2002;17:126–33.PubMedGoogle Scholar
- Halstead SB, Streit TG, LaFontant JG, Putvatana R, Russell K, Sun W, Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission.Am J Trop Med Hyg. 2001;65:180–3.PubMedGoogle Scholar
- Kachur SP, Nicolas E, Jean-François V, Benetiz A, Bloland PB, Saint Jean Y, Prevalence of malaria parasitemia and accuracy of microscopic diagnosis in Haiti, October 1995.Rev Panam Salud Publica. 1998;3:35–9.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. West Nile virus activity—United States, 2001.MMWR Morb Mortal Wkly Rep. 2002;51:497–501.PubMedGoogle Scholar
- Pupo M, Guzman MG, Fernandez R, Llop A, Dickinson FO, Pérez D, West Nile virus infection in humans and horses, Cuba.Emerg Infect Dis. 2006;12:1022–4.PubMedGoogle Scholar
- Komar O, Robbins MB, Guzman Contreras G, Benz BW, Klenk K, Blitvich BJ, West Nile virus survey of birds and mosquitoes in the Dominican Republic.Vector Borne Zoonotic Dis. 2005;5:120–6. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 13, Number 2—February 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Mark E. Beatty, Pediatric Dengue Vaccine Initiative, International Vaccine Institute, SNU Research Park, San 4-8 Bongcheon-7-dong, Kwanak-gu, Seoul, Republic of Korea 151-919;
Top