Volume 31, Number 7—July 2025
Research Letter
Plasmodium knowlesi Malaria in Persons Returning to Israel from Thailand, 2023
Cite This Article
Citation for Media
Abstract
We describe 2 cases of Plasmodium knowlesi malaria in persons from Israel who traveled to Thailand in 2023. One patient, likely infected in northwestern Thailand, might signal geographic expansion into areas not previously associated with human infection. The infection’s rarity in travelers, diagnostic challenges, and potential severity underscore the importance of clinical awareness.
Plasmodium knowlesi, known as the fifth human malaria parasite, is a zoonotic malaria species maintained in a sylvatic cycle involving long-tailed (Macaca fascicularis) and pig-tailed (Macaca nemestrina) macaques and Anopheles (Cellia) leucosphyrus mosquitoes (1). In Thailand, anthroponotic malaria cases have decreased because of intervention programs, whereas P. knowlesi infections have increased, raising public health concerns (2,3). We report 2 cases of P. knowlesi malaria in persons from Israel who traveled to Thailand in 2023. Patient 1 likely acquired malaria in northwestern Thailand and might represent a sentinel case for geographic expansion. Patient 2 was infected in a recognized endemic focus.
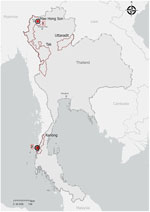
In July 2023, a 25-year-old man (patient 1) sought care for fever, chills, nausea, and retro-orbital pain 1 day after returning from a 7-month trip across Southeast Asia. He spent his final month in northern Thailand, beginning in Chiang Mai and ending with 12 days in Pai, Mae Hong Son Province (Figure 1). He reported jungle trekking, monkey sightings, and no use of malaria prophylaxis. An initial rapid diagnostic test for detecting histidine-rich protein 2 and aldolase was negative, and another for detecting histidine-rich protein 2 and lactate dehydrogenase was weakly positive. A thick smear was initially negative, but a repeat smear 3 hours later revealed nonspeciated Plasmodium. Laboratory findings included leukopenia, thrombocytopenia, increased bilirubin and transaminase levels, and increased C-reactive protein level. TaqMan real-time PCR targeting the 18S rDNA gene performed at the Parasitology Reference Laboratory (https://www.gov.il/he/departments/units/parasite-reference-lab) confirmed P. knowlesi (4,5). PCR and 18S rDNA sequencing yielded a 939-bp fragment with 99.36% identity to P. knowlesi strain H in PlasmoDB (https://plasmodb.org/plasmo/app/record/gene/PKNH_0320900). We treated the patient with artemether/lumefantrine (80 mg/480 mg at 0 and 8 hours and then every 12 hours for a total of 6 doses), and he fully recovered without recrudescence.
In March 2023, we evaluated a 34-year-old man (patient 2), who had spent 6 months in Southeast Asia, for P. knowlesi by using the same molecular diagnostics. He spent his last 2 weeks on Koh Phayam Island in Ranong Province, a recognized endemic area (Figure 1) (2). He reported jungle hiking and macaque contact and did not use prophylaxis. We also treated patient 2 with standard oral artemether/lumefantrine, and he fully recovered.
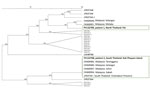
We sequenced the polymorphic C-terminal region of the Pkmsp1 gene to explore genetic diversity. The sequence from patient 1 (northern Thailand) showed 1 synonymous mutation, differentiating it from the P. knowlesi H strain in PlasmoDB (https://plasmodb.org/plasmo/app/record/gene/PKNH_0728900). The closest sequences were from Malaysia, consistent with shared ancestry (6). The translated amino acid sequence matched haplotype H3, which was similar to an amino acid sequence predicted from isolate JF837348 from southern Thailand. The isolate from patient 2 (southern Thailand) was identical to isolate JF837351 from southern Thailand and matched haplotype H2 that is common in the region (6,7) (Figure 2).
The Institutional Review Board (Helsinki Committee, https://www.tasmc.org.il/rd/en/next-unit/helsinki-committee) of Tel Aviv Sourasky Medical Center waived ethical approval. We deposited sequences in GenBank (patient 1: 18S rDNA, accession no. PV123279, and Pkmsp1, accession no. PV132709; patient 2: 18S rDNA, accession no. PV123278, and Pkmsp1, accession no. PV132708).
The likely exposure of patient 1 in Pai or Chiang Mai represents the northernmost documented location of P. knowlesi human infection in Thailand. Previously, the northernmost reported endemic cases were in Tak Province, bordering Myanmar and Uttaradit province, near the Laos border (8). The genetic divergence of the isolate from patient 1 might reflect local parasite diversity or the limited sequence data from northern Thailand and neighboring regions. The Pkmsp1 protein is subject to purifying selection by the host immune system, with surviving variants potentially representing fitter strains. Ongoing genomic surveillance is needed to determine whether patient 1 reflects true local transmission or indicates a broader haplotype range in the region.
Although >280,000 Israelis visited Thailand in 2023, no other confirmed malaria cases were reported among returning travelers, and no other P. knowlesi cases have been reported in Israel in >20 years. In addition to its rarity in travelers, P. knowlesi malaria poses diagnostic challenges because of rapid diagnostic test insensitivity and morphologic similarity to other Plasmodium species, increasing the likelihood of underdiagnosis. Parasitemia levels might be low early in infection, but clinicians should remain alert to the potential for rapid progression to hyperparasitemia, which is a key risk factor for severe malaria attributed to the ability of P. knowlesi to infect all erythrocyte stages and its short asexual replication cycle (9,10). In Malaysia, severe malaria occurred in 6%–9% of cases in district hospitals and up to 29% in tertiary care (9).
Both patients reported here met Centers for Disease Control and Prevention criteria for chemoprophylaxis, having stayed in forested regions near international borders (https://wwwnc.cdc.gov/travel/yellowbook/2024/preparing/yellow-fever-vaccine-malaria-prevention-by-country#6419). The patients’ extended travel and jungle exposure highlight the importance of itinerary-specific pretravel counseling.
In conclusion, P. knowlesi malaria in patient 1 might represent early evidence of zoonotic spillover of P. knowlesi in a new area (northern Thailand). Traveler-based surveillance and molecular tools can provide early warning of geographic shifts in malaria transmission. Heightened clinical awareness and improved diagnostics are essential for timely detection and control.
Dr. Paran is an infectious disease specialist at the Tel Aviv Sourasky Medical Center in Tel Aviv, Israel. Her research interests focus on emerging infectious diseases and geographical medicine.
Acknowledgment
We thank Rona Grossman and David Maimoun for assistance with protein sequence analysis and insightful discussions and Belina Neuberger for valuable language editing services.
References
- Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–24. DOIPubMedGoogle Scholar
- US President’s Malaria Initiative. Thailand malaria profile: FY 2024. Washington (DC): US Agency for International Development; 2024.
- World Health Organization. World Malaria Report 2024: addressing inequity in the global malaria response. Geneva: The Organization; 2024.
- Divis PC, Shokoples SE, Singh B, Yanow SK. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar J. 2010;9:344. DOIPubMedGoogle Scholar
- Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47:975–80. DOIPubMedGoogle Scholar
- Tapaopong P, Chainarin S, Mala A, Rannarong A, Kangkasikorn N, Kusolsuk T, et al. Declining genetic polymorphism of the C-terminus Merozoite Surface Protein-1 amidst increased Plasmodium knowlesi transmission in Thailand. Malar J. 2024;23:342. DOIPubMedGoogle Scholar
- Jongwutiwes S, Buppan P, Kosuvin R, Seethamchai S, Pattanawong U, Sirichaisinthop J, et al. Plasmodium knowlesi Malaria in humans and macaques, Thailand. Emerg Infect Dis. 2011;17:1799–806. DOIPubMedGoogle Scholar
- Narapakdeesakul D, Pengsakul T, Kaewparuehaschai M, Thongsahuan S, Moonmake S, Lekcharoen P, et al. Zoonotic simian malaria parasites in free-ranging Macaca fascicularis macaques and human malaria patients in Thailand, with a note on genetic characterization of recent isolates. Acta Trop. 2023;248:
107030 . DOIPubMedGoogle Scholar - Barber BE, Grigg MJ, Cooper DJ, van Schalkwyk DA, William T, Rajahram GS, et al. Clinical management of Plasmodium knowlesi malaria. Adv Parasitol. 2021;113:45–76. DOIPubMedGoogle Scholar
- Lee WC, Cheong FW, Amir A, Lai MY, Tan JH, Phang WK, et al. Plasmodium knowlesi: the game changer for malaria eradication. Malar J. 2022;21:140. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: June 18, 2025
Table of Contents – Volume 31, Number 7—July 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Tamar Grossman, Reference Parasitology Laboratory, Public Health Laboratories–Jerusalem, Israeli Ministry of Health, Yaakov Eliav 9, Jerusalem 9134302, Israel
Top