Volume 13, Number 6—June 2007
Research
Bovine Spongiform Encephalopathy and Spatial Analysis of the Feed Industry
Cite This Article
Citation for Media
Abstract
In France, despite the ban of meat-and-bone meal (MBM) in cattle feed, bovine spongiform encephalopathy (BSE) was detected in hundreds of cattle born after the ban. To study the role of MBM, animal fat, and dicalcium phosphate on the risk for BSE after the feed ban, we conducted a spatial analysis of the feed industry. We used data from 629 BSE cases as well as on use of each byproduct and market area of the feed factories. We mapped risk for BSE in 951 areas supplied by the same factories and connection with use of byproducts. A disease map of BSE with covariates was built with the hierarchical Bayesian modeling methods, based on Poisson distribution with spatial smoothing. Only use of MBM was spatially linked to risk for BSE, which highlights cross-contamination as the most probable source of infection after the feed ban.
In France, meat-and-bone meal (MBM) has been banned from cattle feed since July 30, 1990. However, through January 1, 2007, 957 cases of bovine spongiform encephalopathy (BSE) have been detected in cattle born after the ban. These cases provide evidence that BSE control has not been entirely effective, which poses a concern because BSE is a zoonotic disease, a source of variant Creutzfeldt-Jakob disease (vCJD). Until now, 158 definite or probable cases of vCJD in humans have been detected in the United Kingdom (www.cjd.ed.ac.uk/figures.htm, consulted March 12, 2007) and 21 in France (www.invs.sante.fr/recherche, consulted March 12, 2007). The risk for humans is controlled by removing specified risk materials from human consumption (since 1996 in France, later in other European countries) and testing all cattle at the abattoir with a rapid test (since 2001 in continental European Union). However, these measures are expensive. Achieving 100% control of the spread of BSE is a major challenge, important for human health but limited by economic constraints.
The main hypothesis concerning the source of infection in cattle born after the MBM ban still involves MBM; the BSE agent may have entered cattle feed by cross-contamination with feed for monogastric species (pigs and poultry) in which MBM was still authorized until November 2000. Cross-contamination could have occurred within factories, during feed delivery to the farm, or on mixed farms that have cattle and pigs or poultry. This hypothesis is supported by the finding of MBM traces in cattle feed (1) as well as by epidemiologic studies that showed a spatial link between density of monogastric species and risk for BSE (2–7).
Another hypothesis, however, suggests the role of other animal byproducts such as fat and dicalcium phosphate (DCP) derived from bones, which were not prohibited in cattle feed before 2000. Such components might have been contaminated by the BSE agent during cattle slaughter (1). Clauss et al. (5) found a statistically higher use of milk replacers (which contain animal fat) for calves on BSE-affected farms in Germany, and the same type of association was observed in France for scrapie in sheep (8).
More knowledge about these factors is critical for the management of the BSE risk, as the BSE epidemic decreases and pressure increases to release progressively more stringent control measures. Risk for BSE was spatially heterogeneous in France for the infected cattle born after the ban (9), meaning that the source of infection might be spatially heterogeneous. If animal byproducts were a source of BSE for cattle born after the ban, we would expect a higher risk for BSE in areas with higher use of those animal byproducts in feed. We therefore investigated geographic variations in the use of animal byproducts in feed factories (MBM for monogastric species, animal fat and animal DCP for cattle) and explored their spatial link with risk for BSE in the market areas of the factories.
Data Collection
BSE Cases
BSE cases are recorded in the BSE database of the Agence Française de Sécurité Sanitaire des Aliments (AFSSA) in Lyon, France. The period we considered for case detection was July 1, 2001, through December 31, 2005. This choice enabled us to obtain precise and comparable data because during this period detection of BSE was based on the mandatory reporting system and on the comprehensive active surveillance program (10,11); these 2 systems are complementary and ensure screening of every bovid >24 months of age, dead or slaughtered.
Because feed produced before the ban was not recalled and to account for possible stocks of feed, our study was restricted to BSE-infected cattle born after January 1, 1991. As shown in previous studies (12,13), the source of infection for cattle born after the ban and for those born after a complementary reinforced ban implemented in 1996 (www.agriculture.gouv.fr/esbinfo/esbinfo.htm, consulted September 7, 2006) appear to be the same. Therefore, to increase the statistical power of the study, we combined infected cattle born after the ban with those born after the complementary reinforced ban.
BSE cases considered in the analysis were in clinically suspected animals confirmed at AFSSA with Western blot or immunochemical tests and in animals with positive test results (same techniques) among the entire cattle population tested within the active surveillance program (11). The location of the BSE-infected cattle was defined by the center of the commune (French community, 36,582 units, average 15 km²) of the farm in which the animal had been raised during the 6–12 months after birth; according to modeling results, this period corresponds to the highest risk for infection (14,15).
Cattle Characteristics and Demographics
The background population was based on demography of female adult bovids available at the canton level (3,705 units, average 150 km²). Data were obtained from the Agricultural Census 2000 (CD-ROM, edited by Agreste, 251 rue de Vaugirard, 75732 Paris, France). Because BSE incidence varies according to production type, the cattle population was divided into dairy and beef (3,16,17); incidence was defined by breed of the animal or production type of the farm (when breed was not recorded or when animal was a mixed breed).
Factory Data
From March 2004 through June 2005, the Ministry of Agriculture (Direction Générale de l’Alimentation) used a questionnaire designed by the authors to investigate factories that produced compound feed for cattle. The period of interest for the questionnaire was January 1, 1991, through November 2000 (date of the total ban of MBM and byproducts in feed for all species). The 4 characteristics used in the analysis were market area (geographic area in which the factory was delivering feed), use of MBM in feed for monogastric species, use of animal fat in cattle feed, and use of animal DCP in cattle feed. Factories were located by the commune center. Canton perimeters and commune centers were provided by GEOFLA France Métropolitaine (Institut Géographique National, Paris, France; version 6; 2002).
Mapping Use of Byproducts in Feed Factories
Each factory had a unique market area; market areas varied in size and partly overlapped. To handle this complex situation, we listed all factories that delivered feed in each canton; then we used ArcView GIS (ESRI Inc. Redlands, CA, USA) to perform a spatial aggregation of all cantons that used the same set of factories and called this a delivery area. From 327 market areas, this process divided the territory into 943 delivery areas. Consequently, each delivery area could be considered homogeneous for the risk related to factories. For each byproduct (MBM, animal fat, animal DCP), exposure of cattle in a given delivery area was estimated by the proportion of factories that used this byproduct in the area (number of factories that used the byproduct divided by total number of factories in the area). The exposure of each area to each byproduct was then classified in quintiles and mapped. Use of quintiles enabled us to have a central class. To improve the legibility of the map, we smoothed it by using a spatial interpolation (kriging) of the values of the exposure in the delivery areas.
BSE Risk and Spatial Link with Use of Byproducts
To assess the relative risk for BSE—comparing risk in a given delivery area with average risk in the nation—we modeled the distribution of the number of BSE cases, taking into account the bovine demography in each area and the geographic adjacency of areas (18–21). The method is explained in detail in the online Technical Appendix, available at www.cdc.gov/EID/13/6/867-app.htm. A basic model (model 0) without covariate (factor explaining the risk) was assessed to represent the risk for BSE in each area, following a method used previously (9); the relative risk for BSE in the area was then classified in quintiles, as was risk for exposure.
The crude link between exposure to each byproduct and risk for BSE was assessed by the crossing of the 5 levels of the relative risk for BSE and the 5 levels of the exposure to MBM, animal fat, and animal DCP, respectively, which was reported in a 5×5 array table. The 25 cells of the table were classified in 5 groups on which the mapping of the crude link was based. Groups 1 and 2 represented a concordant relationship between the relative risk for BSE and exposure, i.e., high exposure with relative risk for BSE >1 on the one hand, and low exposure with relative risk for BSE <1 on the other. Groups 3 and 4 represented a discordant relationship, i.e., high exposure with relative risk <1 and the converse, and group 5 was intermediate. Furthermore, the crude link between the use of each byproduct in the area and the relative risk for BSE was tested with a Spearman rank correlation unilateral test.
Finally, the adjusted link between exposure to each byproduct and risk for BSE was assessed by including covariates in the hierarchical Bayesian model. We successively incorporated all covariates with a significant crude link with BSE, following a method used in a previous work (22) and shown in detail in the online Technical Appendix.
Sensitivity Analysis Regarding Missing Data
To test the effect of possible bias due to missing data, we conducted a sensitivity analysis. We assumed that missing data were randomly distributed for animal fat and animal DCP, which were still authorized in cattle feed until November 2000. For MSM, however, we hypothesized that lack of answer could be a way to escape the question; because MBM was prohibited for cattle, cross-contamination of cattle feed with MBM in feed for monogastric species should in fact be the responsibility of manufacturers that were not successful in controlling such a risk within their factories. So we tested a fictitious situation under a worst-case scenario: all missing data about MBM would be related to use of MBM in feed for monogastric species. The crude link between the use of MBM and the relative risk for BSE was mapped under this scenario and tested with a Spearman rank correlation unilateral test.
Use of Byproducts in Feed Factories
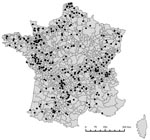
We identified 327 factories that were producing compound feed for cattle during the study period (Figure 1). From the market areas, we divided the territory into 943 delivery areas (Figure 1), 7–9,734 km² (average 578 km²); 4–33 (average 16) factories delivered in a given area. Factories that did not answer questions on a given risk were excluded from analysis, except for the sensitivity analysis. The final sample with complete data was composed of 255 factories for the risk concerning MBM, 248 for animal fat, and 220 for animal DCP. In the delivery areas, the proportions of factories that used animal byproducts (Figure 2) were 16.7%–71.4% for MBM, 5.3%–68.8% for animal fat, and 13.3%–88.9% for animal DCP.
Relative Risk for BSE and Crude Link with Factory Variables
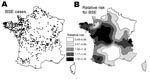
From July 1, 2001, through December 31, 2005, 525 BSE-infected cattle born after the ban and 104 born after the complementary reinforced ban were detected in France (Figure 3A). Among these 629, 505 were detected in the dairy cattle population (4.2 million) and 124 in the beef cattle population (4.3 million). The relative risk for BSE, based on the model without covariate (Table, model 0), varied between 0.49 and 2.29, depending on the area (Figure 3B). The maps of the crude link between relative risk for BSE and factory variables (Figure 4) show the concordant and discordant areas between the relative risk for BSE and use of each byproduct.
According to the Spearman rank correlation unilateral test, only use of MBM (ρ = 0.43, p<0.001) and use of animal fat (ρ = 0.39, p<0.001) appeared significantly linked to the relative risk for BSE. For MBM, the link remained the same (ρ = 0.43, p<0.001) when we performed the sensitivity analysis and replaced missing data with “yes”; we therefore eliminated missing data from the analysis. Because the link between animal DCP and the relative risk for BSE was not significant, this covariate was not incorporated into the disease mapping models.
Disease Mapping Models
The Table presents the set of models assessed with the covariates MBM and animal fat. We deduced from the lowest deviance information criterion (DIC) value of models 1 (ΔDIC = –5.0) and 3 (ΔDIC = –3.0) that only use of MBM significantly influenced the relative risk for BSE. The results of conformity tests H0 : β1 = 0 were significant (p<0.03). The model quantified the effect of the risk factor: the risk for BSE was 3.8× higher in a delivery area in which 100% of the factories used MBM compared with another area in which none of the factories used MBM.
We used spatial analysis to explore the link between use of 3 byproducts (MBM, animal fat, animal DCP) in factories that produced cattle feed and the relative risk for BSE for animals born after the ban of MBM in cattle feed in France. Among 327 factories, questionnaires were incomplete for 72 (22%) for the use of MBM, 79 (24%) for animal fat, and 107 (33%) for animal DCP. Our hypothesis for missing data about animal DCP was that manufacturers did not know the answer because they often bought premix with preincorporated minerals. Therefore, lack of responses should not be biased and should not affect the analysis. We applied the same hypothesis to absence of information bias for the use of animal fat because this byproduct was allowed in cattle feed and manufacturers would have no reason to hide data. For MBM, the hypothesis of a possible information bias (because MBM was banned from cattle feed) was tested in a sensitivity analysis using a worst-case scenario; this scenario did not change the result, so a possible information bias, if any, should not have modified the results. The huge regional differences in the proportion of factories using MBM, animal fat, and animal DCP might have different explanations, including the local supply, which is linked to local production or import availability, and the differential interest in using each of these compounds for feed for different species whose densities vary in this French territory.
The main result of the spatial analysis provides evidence of a significant adjusted spatial link between factory use of MBM for monogastric species and the relative risk for BSE. This result favors the effect of cross-contamination of cattle feed with MBM-containing feed for monogastric species as a source of BSE for cattle born after the ban of MBM. A recent epidemiologic study in France (23) clearly showed that cattle that consumed feed from factories were at risk for BSE after the feed ban; it also showed that mixed farms were at a higher risk for BSE, which indicates that cross-contamination has possibly occurred on farms (by feeding monogastric-species feed to bovines). These findings are in agreement with our results; both studies complement each other and raise the question of effectiveness of the ban that was initially restricted to bovines and belatedly extended to other species to reduce cross-contamination.
Our study did not implicate animal fat as a source of infection. However, we cannot exclude a minor effect, which would be impossible to prove given the power of the study. Animal fat is considered potentially infectious because of the solubility of prions (24,25) and the possible contamination with protein impurities by contact with other infectious materials at the slaughterhouse. Animal fat is incorporated in cattle feed in milk replacer and in proprietary concentrates. Clauss et al. (5) identified milk replacer as a potential risk factor for BSE in Germany, of importance comparable to proprietary concentrates; the case-control study carried out in France (23) also found an effect of consumption of milk replacer, but to a lesser extent. Regardless, distinguishing the specific effect of milk replacer and proprietary concentrate in these studies was difficult.
Concerning animal DCP, our study showed no effect of its use in compound feed for cattle; however, we did not take into account mineral and vitamin compounds fed to cattle, which can incorporate animal DCP as well. Our results agree with those of the case-control study (23), which did not provide evidence that use of mineral and vitamin compounds affect risk for BSE; the authors considered that the implication of animal DCP as a source of BSE, if it existed, should have been marginal. In contrast, a risk analysis by the European Food Safety Agency (www.efsa.europa.eu/en/science/biohaz/biohaz_opinions/1440.html, consulted 7 September 2006) highlighted the potential role of animal DCP in cattle infection, which might be the same order of magnitude as the residual risk from cross-contamination with MBM. Our results do not support this assessment; further studies would be useful.
Our spatial study highlighted the role of MBM as a source of BSE after the ban of MBM for cattle, through cross-contamination in feed factories. If we exclude deliberate use of MBM in feed for cattle (banned since 1990), our findings indicate that feed manufacturers did not implement sufficient measures to avoid cross-contamination during feed processing. Different key points were identified by the French Ministry of Agriculture (B. Thiebot and X. Delomez, pers. comm.) as minimizing risk for cross-contamination. Some can be implemented quickly, such as the sequence of processing, namely, banning the processing of feed for monogastric species just before feed for cattle. However, others are more difficult to implement, such as automatic computation of formula, automatic computation of the sequence of production, and automatic incorporation of unsold products in feed. The ultimate way to eliminate cross-contamination is to have a complete partition between the feed-processing chains dedicated to monogastric species and to ruminants, a huge investment for the feed industry with low profit margins. Given the situation in the field, the results of our study indicate that the total ban of MBM for farm animals in November 2000 was essential for controlling the spread of BSE.
In the current context of the decreasing epidemic, economic pressure is increasing to release the ban of MBM in feed for monogastric species. The prerequisite, from an animal and human health perspective, is 100% efficient control of the risk for cross-contamination at the factory level and elsewhere. Releasing any control measure would need comprehensive cooperation within the feed industry to adapt their production units, which cannot be achieved in the short term.
Dr Paul is a researcher at the French National Institute for Agricultural Research. Her primary research interest is spatial epidemiology and health geography applied to emerging animal diseases.
Acknowledgment
The study would have not been possible without the collaboration of the county veterinary services that collected data from the feed industries. We also thank V. Cespedes for data management, J. de Goer for computer development and database management, and A.-S. Martel for data input. We are indebted to X. Delomez and B. Thiebot for their valuable expertise on the feed industry.
References
- Agence Française de Sécurité Sanitaire des Aliments. Les risques sanitaires liés aux différents usages des farines et graisses d'origine animale et aux conditions de leur traitement et de leur élimination. Maisons-Alfort (France): l’Agence; 2001. p. 1–200.
- Ducrot C, Calavas D. Epidémiologie de l’encéphalopathie spongiforme bovine et de la tremblante. In: Dodet B, Cathala F. Les maladies humaines et animales à prions. Paris: Elsevier; 2002: p.47–60.
- Wilesmith JW, Wells GAH, Cranwell MP, Ryan JBM. Bovine spongiform encephalopathy: epidemiological studies. Vet Rec. 1988;123:638–44.PubMedGoogle Scholar
- Wilesmith JW, Ryan JBM, Stevenson MA, Morris RS, Pfeiffer DU, Lin D, Temporal aspects of the epidemic of bovine spongiform encephalopathy in Great Britain: holding-associated risk factors for the disease. Vet Rec. 2000;147:319–25. DOIPubMedGoogle Scholar
- Clauss M, Sauter-Louis C, Chaher E, Pottgiesser C, Goebel S, Selhorst T, Investigations of the potential risk factors associated with cases of bovine spongiform encephalopathy in Bavaria, Germany. Vet Rec. 2006;158:509–13. DOIPubMedGoogle Scholar
- Wilesmith JW, Ryan JBM, Hueston WD. Bovine spongiform encephalopathy: case-control studies of calf feeding practices and meat and bone meal inclusion in proprietary concentrates. Res Vet Sci. 1992;52:325–31. DOIPubMedGoogle Scholar
- Doherr MG, Hett AR, Rufenacht J, Zurbriggen A, Heim D. Geographical clustering of cases of bovine spongiform encephalopathy (BSE) born in Switzerland after the feed ban. Vet Rec. 2002;151:467–72. DOIPubMedGoogle Scholar
- Philippe S, Ducrot C, Roy P, Remontet L, Jarrige N, Calavas D. Sheep feed and scrapie, France. Emerg Infect Dis. 2005;11:1274–9.PubMedGoogle Scholar
- Abrial D, Calavas D, Jarrige N, Ducrot C. Spatial heterogeneity of the risk of BSE in France following the ban of meat and bone meal in cattle feed. Prev Vet Med. 2005;67:69–82. DOIPubMedGoogle Scholar
- Calavas D, Ducrot C, Baron T, Morignat E, Vinard JL, Biacabe AG, Prevalence of BSE in western France by screening cattle at risk: preliminary results of a pilot study. Vet Rec. 2001;149:55–6. DOIPubMedGoogle Scholar
- Calavas D, Ducrot C. L’ESB en France: synthèse sur l'évolution de l’épizootie à partir des données disponibles au 1er janvier 2003. Maisons-Alfort (France) : Agence Française de Sécurité Sanitaire des Aliments. Rapport; 2003. p. 1–16.
- Ducrot C, Abrial D, Calavas D, Carpenter T. A spatio-temporal analysis of BSE cases born before and after the reinforced feed ban in France. Vet Res. 2005;36:839–53. DOIPubMedGoogle Scholar
- Jarrige N, Ducrot C, Lafon D, Thiebot B, Calavas D. Potential sources of infection for BSE cases born in France after 1996. Vet Rec. 2006;159:285–6. DOIPubMedGoogle Scholar
- Donnelly CA. Likely size of the French BSE epidemic. Nature. 2000;408:787–8. DOIPubMedGoogle Scholar
- Supervie V, Costagliola D. The unrecognised French BSE epidemic. Vet Res. 2004;35:349–62. DOIPubMedGoogle Scholar
- Ducrot C, Roy P, Morignat E, Baron T, Calavas D. How the surveillance system may bias the results of analytical epidemiological studies on BSE: prevalence among dairy versus beef suckler cattle breeds in France. Vet Res. 2003;34:185–92. DOIPubMedGoogle Scholar
- Morignat E, Ducrot C, Roy P, Baron T, Vinard JL, Biacabe AG, Targeted surveillance to assess of BSE in high risk populations in western France and the associated risk factors. Vet Rec. 2002;151:73–7. DOIPubMedGoogle Scholar
- Elliott P, Wakefield JC, Best NG, Best DJ. Spatial epidemiology: methods and applications. In: Elliott P, Wakefield JC, Best NG, Best DJ, editors. Spatial epidemiology. London: Oxford University Press; 2000. p. 3–14.
- MacNab YC. Hierarchical Bayesian modeling of spatially correlated health service outcome and utilization rates. Biometrics. 2003;59:305–16. DOIPubMedGoogle Scholar
- Richardson S, Best NG. Bayesian hierarchical models in ecological studies of health-environment effects. Environmetrics. 2003;14:129–47. DOIGoogle Scholar
- Banerjee S, Carlin BP, Gelfand AE. Hierarchical modeling and analysis for spatial data. London: Chapman & Hall; 2004: p. 99–128.
- Abrial D, Calavas D, Jarrige N, Ducrot C. Poultry pig and the risk of BSE following the feed ban in France—a spatial analysis. Vet Res. 2005;36:615–28. DOIPubMedGoogle Scholar
- Jarrige N, Ducrot C, Cazeau G, Cazeau G, Morignat E, La Bonnardière C, Case-control study on feed risk factors for BSE cases born after the feed ban in France. Vet Res. 2007. In press. DOIPubMedGoogle Scholar
- Appel TR, Riesner D, von Rheinbaben F, Heinzel M. Safety of oleochemical products derived from beef tallow or bone fat regarding prions. Eur J Lipid Sci Technol. 2001;103:713–21. DOIGoogle Scholar
- Appel T, Wolff M, von Rheinbaben F, Heinzel M, Riesner D. Heat stability of prion rods and recombinant prion protein in water, lipid and lipid-water mixtures. J Gen Virol. 2001;82:465–73.PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 13, Number 6—June 2007
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Christian Ducrot, INRA, Unité d’Épidémiologie Animale UR346, Département de Santé Animale, 63122 Saint Genès Champanelle, France
Top