Volume 14, Number 8—August 2008
Research
Diverse Contexts of Zoonotic Transmission of Simian Foamy Viruses in Asia
Cite This Article
Citation for Media
Abstract
In Asia, contact between persons and nonhuman primates is widespread in multiple occupational and nonoccupational contexts. Simian foamy viruses (SFVs) are retroviruses that are prevalent in all species of nonhuman primates. To determine SFV prevalence in humans, we tested 305 persons who lived or worked around nonhuman primates in several South and Southeast Asian countries; 8 (2.6%) were confirmed SFV positive by Western blot and, for some, by PCR. The interspecies interactions that likely resulted in virus transmission were diverse; 5 macaque taxa were implicated as a potential source of infection. Phylogenetic analysis showed that SFV from 3 infected persons was similar to that from the nonhuman primate populations with which the infected persons reported contact. Thus, SFV infections are likely to be prevalent among persons who live or work near nonhuman primates in Asia.
Human society critically influences the ecologic contexts in which the transmission of infectious agents between species occurs (1,2). In developing countries, economic growth and new infrastructure have transformed the human–animal interface, facilitating the emergence of previously unrecognized zoonotic diseases. Nowhere is this more evident than in South and Southeast Asia, where the world’s densest human populations are situated close to some of the planet’s richest reservoirs of biodiversity (3). Nonhuman primates figure prominently as potential sources of emerging human pathogens (4).
Asian cultures have long traditions of venerating nonhuman primates, and in many Asian communities nonhuman primates (particularly macaques and langurs) are woven into the fabric of everyday life (5–8). Nonoccupational interspecies contact occurs in urban settings, parks and religious sites, settings where nonhuman primates are kept as pets or performance animals, animal markets, and zoos; it also occurs during bushmeat hunting and consumption (9,10).
The first 2 contexts listed merit particular attention because they represent nonoccupational situations in which cross-species disease transmission can occur, and they represent settings in which large numbers of humans and nonhuman primates come into contact. Urban nonhuman primates are found in towns and densely populated cities throughout South and Southeast Asia, where their population may reach several thousands (5,11). This urban niche frequently and increasingly brings them into close contact with humans, as much of their food supply is provided by humans, formally or informally (as when nonhuman primates raid homes or scavenge for refuse). Temple monkeys are free-ranging in parks and religious sites in South and Southeast Asia and have lived commensally with humans for centuries at these sites, some of which have become international tourist destinations.
Simian foamy viruses (SFVs) comprise a subfamily of simian retroviruses that are ubiquitous in nonhuman primates. Ancient and well adapted, SFVs have coevolved with their nonhuman primate hosts for >30 million years (12). Once acquired, SFV infections are lifelong and do not seem to cause disease in their natural hosts (13). Nearly all captive and free-ranging macaques have acquired SFV infection by adulthood (14,15).
Studies have demonstrated that humans who are occupationally exposed to captive or free-ranging nonhuman primates can acquire SFV infection, although the number of known SFV-infected humans is small. At-risk populations include veterinarians; laboratory, temple, and zoo workers; pet owners; and bushmeat hunters (16–20). SFV prevalence in these populations is 1%–6%. The possibility of human-to-human transmission has been investigated among a small number of SFV-positive persons and their spouses and/or children. To date, no evidence of human-to-human transmission of SFV has been found (16,21).
Because of the close association of humans and nonhuman primates in Asia, most of which occurs in nonoccupational settings, we examined a large number of persons from several countries for evidence of SFV infection. All participants were asked about their past interactions with nonhuman primates, the species and population of the nonhuman primates with which they interacted, the behavioral contexts of each interaction, and the kinds of injuries, if any, inflicted. Our aim was to detect nonhuman primate–to-human SFV transmission and to learn about the behavioral contexts in which it occurs.
Study Sites and Populations
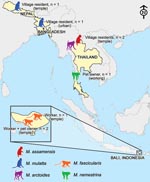
Our data were gathered over 7 years, and our sample totaled 305 persons (172 men, 133 women). Study sites, selected for their known human–nonhuman primate contact, were located in 4 countries in South and Southeast Asia: Thailand, Indonesia, Nepal, and Bangladesh. The seroprevalence of SFV among the nonhuman primates at these sites has been reported (9,15,17).
In Thailand, 211 persons were interviewed and sampled: 8 workers from a zoo in northern Thailand in 2002 and 203 persons at temples, nonhuman primate pet owners, bushmeat hunters, and urban residents from 9 sites in 2004–05. In Indonesia, biological samples and demographic and exposure data were collected from 74 temple workers at 2 sites in Bali: AK in 2000 (n = 56) and UB in 2003 (n = 18). In Nepal in 2003, 9 persons who lived and/or worked at the Swoyambhu Temple in Kathmandu were sampled; this World Heritage site is home to >400 free-ranging rhesus (Macaca mulatta). In Bangladesh, where for decades ≈200 rhesus monkeys have ranged freely in the village of DH, northeast of Dhaka, 11 villagers were sampled and interviewed in 2007.
Protocols for human subject recruitment, biological sample collection, storage and handling, and collection of ethnographic/epidemiologic data have been described (17). Questionnaires and laboratory databases were analyzed by using NCSS 2004 (Kaysville, UT, USA) databases. Protocols for obtaining questionnaire data and biological samples were reviewed and approved by the University of Washington Human Subjects Institutional Review Board (02-5676-C06).
SFV Assays
A bioplex whole-virus multiplex flow cytometric assay was used for SFV antibody screening. SFV was conjugated to beads as previously described for simian retrovirus, simian T-cell leukemia virus, simian immunodeficiency virus, and Cercopithecine herpesvirus 1 (22). The results were validated by using plasma from known SFV-positive and SFV-negative monkeys (as determined by immunofluorescence assay). The ELISA using bacterially expressed, purified glutathione S-transferase (GST) and GST-Gag has been described (23). For further testing, we conducted a Western blot (WB) assay with SFV-infected or SFV-noninfected cell lysates; the SFV used was isolated from an M. fascicularis housed at the University of Washington. Viral bands were detected by using the TMB reagent (3,3′,5,5′-tetramethylbenzidine; Promega, Madison, WI, USA). This assay has been previously described (15). Each assay used a strongly positive human serum (HCM2) and negative serum sample from a person who had never been exposed to a nonhuman primate.
Molecular and Phylogenetic Analyses
DNA was extracted from blood samples by using QIAamp Blood Mini Kits (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. For PCRs, the primers and conditions described by Schweizer and Neumann-Haefelin were used for pol (24), and those by Jones-Engel et al. (17) were used for mitochondrial sequences, with the following modifications: an annealing temperature of 52°C was used for 25 cycles in round 1 and for 29 cycles in round 2. For gag PCR, the following oligonucleotide primers were used: round 1 forward primer 5′-AGGATGGTGGGGACCAGCTA-3′, reverse primer 5′-GCTGCCCCTTGGTCAGAGTG-3′; round 2 forward primer 5′-CCTGGATGCAGAGCTGGATC-3′, reverse primer 5′-GAG GGAGCCTTTGTGGGATA-3′. The PCR conditions for gag and pol PCR were identical. All PCR runs included tubes containing water and noninfected human DNA as negative controls. DNA was checked for integrity by using mitochondrial DNA primers. Purified PCR fragments were cloned from round 2 into pCR2.1-TOPO by using the TOPO TA Cloning Kit for Sequencing (Invitrogen, Carlsbad, CA, USA). For each clone, 3–6 colonies were picked and purified-DNA sequenced. Sequences were analyzed by using Sequencher 4.7 (Gene Codes Corporation, Ann Arbor, MI, USA). For pol, 425 bp were compared; for gag, 1,125 bp were compared. Trimmed sequences were analyzed by using BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi) and aligned, and neighbor-joining trees (25) were estimated by using the Tajima and Nei model (26). Bootstrap values (1,000 replicates) are represented as percentages. Positions containing gaps and missing data were not considered in the analysis. Phylogenetic analyses were conducted in MEGA (27). Identical results were obtained with MrBayes (28) (analyses not shown) under the Hasegawa, Kishino, and Yano substitution model (28). In those analyses a search was performed with 1 million generations, and the first 100,000 trees were discarded in the burn-in.
Nucleotide Sequence Accession Numbers
The gag and pol gene sequences reported here were deposited in GenBank under the following accession nos.: AK04gag EU448349, AK04pol EU448363, AK19gag EU448350, AK19pol EU448364, AK23gag EU448351, AK23pol EU448365, BGH4 gag EU450664, HAD3 gag EU450665, HAD38pol EU448341, HAD3pol EU448342, MBG11gag EU448344, MBG13gag EU448345, MBG14gag EU448346, MBG4gag EU448343, MBG7gag EU448347, MBG8gag EU448348, SFVfasWgag EU448357, SM44gag EU448353, SM44pol EU448358, SM46pol EU448359, SM49gag EU448354, SM49pol EU448360, SM61gag EU448355, SM61pol EU448361, SM62gag EU448356, SM62pol EU448362, UB1pol EU448366, UB3gag EU448352, and UB3pol EU448367. SFVmulO is listed under accession no. DQ120937.
Demographic Data (Table 1)
Persons ranged from 18 to 80 years of age. Their context of contact with nonhuman primates was defined as the predominant form of contact at the time of the study. Some persons reported other past contexts of contact. For example, several of the 23 bushmeat hunters, all from the same village in Thailand, had previously worked at a park where free-ranging nonhuman primates were the main attraction, and a few of the temple workers in Bali and Thailand reported having previously owned pet nonhuman primates.
SFV Assays (Table 2)
Of 305 serum samples analyzed, 211 samples from Thailand were initially screened with bioplex at the Washington National Primate Research Center (22), and 146 of these samples had negative results. The remaining 65 samples from Thailand and all 94 samples from Nepal, Indonesia, and Bangladesh were screened by ELISA by using GST control antigen and GST-Gag fusion protein. Of these 159 samples, reactivity of 25 exceeded GST background on ELISA, and these were further tested with WB by using SFV-infected or SFV-noninfected tissue culture cell lysates. The major reactive viral protein is the structural protein Gag. Some foamy virus–infected serum samples also react with the viral accessory protein Bet. A total of 8 (2.6%) human samples were confirmed positive by using SFV-infected tissue culture cell or noninfected cell control lysates, which all react with the Gag protein. Gag appears as a characteristic doublet of 68 and 71 kDa (Figure 1, samples 2–9). Antibody to Bet could be detected only in HCM2, HAD3, and NH2. Although reactivity of HMS14 antiserum is weak, this serum was able to neutralize SFV but not the chimpanzee-derived primate foamy virus, which confirmed infection (data not shown). All other human serum samples tested were negative for all viral proteins. Two negative examples are shown in Figure 1: HCJ7, which yielded the same background proteins in noninfected and infected lysate, and BGH1, which did not react with any proteins. Human serum samples were also tested by WB by using GST and GST-Gag protein (15). However, because many of the human samples reacted with GST protein, the recombinant protein WB assays were generally inconclusive (data not shown).
Prevalence of Bites
No statistical differences in bite exposures were detected between men and women (χ2 = 0.009, p = 0.924, degrees of freedom = 1) or among age groups (χ2 = 7.678, p = 0.1043, degrees of freedom = 4). Bites were less common among bushmeat hunters (0%) and persons who lived and/or worked at monkey temples (25.6%) than among those who were exposed to urban (57.9%) and pet monkeys (52.4%). Splashes of body fluids onto mucosa were reported by nearly one fourth (24.9%) of the study population and scratches by 38.4%. Overall, 63.6% of the total population reported being exposed to nonhuman primate body fluids through a bite, scratch, or splash onto mucosa.
Nonhuman Primate Contacts Reported by SFV-positive Persons (Table 3)
Thailand
At the time of sampling, HCM2, a farmer from central Thailand, was 56 years of age. Since 23 years of age, he had trained 8 pig-tailed macaques (M. nemestrina) to harvest coconuts. At the time of data collection, he had 3 working M. nemestrina that he kept in his compound and transported to the fields on his motorbike. He reported having received several scratches and 2 bleeding bites (hand and arm) over the years. The bites were treated with traditional medicines.
At the time of sampling, HMS14 was 44 years of age. She sold food at a Buddhist temple in northern Thailand and had worked and lived in the area for 30 years. Wild assamese macaques (M. assamensis) ranged freely through the temple grounds, commonly entered nearby homes in search of food, and frequently received food from monks and visitors to the temple. HMS14 reported that M. assamensis came into her home daily to raid food bins. In 1999, a pet female stump-tailed macaque (M. arctoides) was brought to the temple and released. HMS14 had repeated physical contact (but no bites or scratches) with this released pet macaque, which was often present at her food stall. HMS14 reported that on 3 separate occasions in 2004 she was scratched by free-ranging M. assamensis and that the scratches were deep enough to bleed.
HMS50, a 43-year-old laborer who had lived in a village in northern Thailand for 33 years, reported that he came to the Buddhist temple several times a week and that M. assamensis entered his home, near the temple, a few times a year in search of food. He reported no bites, scratches, or mucosal splashes. He did report that he fed the M. assamensis at the temple site.
Indonesia
HAD3, a 58-year-old man, worked at a Hindu temple site in central Bali, where free-ranging long-tailed macaques (M. fascicularis) were an attraction for domestic and international tourists. He also reported that he had previously owned 2 pet M. fascicularis. He reported having received >5 bleeding bites to his hands from his pet macaques and 1 bleeding bite and multiple scratches from macaques at the temple site. He did not seek medical treatment for the bites or scratches.
HAD38, a 32-year-old woman, had worked as a tourist guide at the same temple site as HAD3. She reported having received 2 bleeding bites and a bleeding scratch from the macaques within 1 year of working at this site. She applied antiseptic to her injuries.
HUB7, a 35-year-old temple worker at a Hindu temple in central Bali, reported that during his 2.5 years of work there he had been bitten 4 times by free-ranging M. fascicularis, once each on the hand, arm, leg, and buttock. All bites were severe enough to cause bleeding. He washed each wound with water and sought medical care, which included a tetanus vaccine and antimicrobial drugs, for the bite on his arm. He reported having been scratched only 1 time. He also had touched a pet M. fascicularis owned by a family in his village but had never been bitten or scratched by that macaque.
Nepal
NH2, a 36-year-old woman, lived immediately adjacent to the Swoyambhu Temple in Kathmandu and occasionally worked there as a cleaner. She had been bitten 1 time on her middle finger by one of the temple’s free-ranging rhesus macaques (M. mulatta). The wound was washed with water, and she was treated with a rabies vaccination and antimicrobial drugs at a local clinic. She denied having ever been scratched.
Bangladesh
BGH4, a 19-year-old housewife, was born in the central Bangladeshi village in which she was sampled. When she was 4 years old, she was severely bitten on her left calf by one of the M. mulatta that ranged freely through the village. She did not recall whether she had received any medical treatment. She did not report any other physical contact with nonhuman primates, though she did comment that the local macaques often entered her house in search of food, leaving urine and feces.
Phylogenetic Analyses of SFV Sequences
We derived SFV sequences from the peripheral blood DNA of 3 SFV-infected persons: BGH4, HAD3, and HAD38. We were able to amplify mitochondrial DNA from the DNA sample of another person (HCM2) from which no SFV sequences could be obtained. We have no evidence that DNA obtained from the other 4 human blood samples was of good quality (data not shown). We obtained gag sequences from BGH4 (Figure 2, panel A), gag and pol sequences from HAD3 (Figure 2, panels B, C), and pol sequences from HAD38 (Figure 2, panel C). SFV sequences from humans were compared with those from macaques of the group with which the person had been in contact and with those from other macaques of the same species but different geographic origin (Figure 2, panel A, M. mulatta; Figure 2, panels B, C, M. fascicularis).
SFV from BGH4 clustered most closely with SFV from 4 M. mulatta from her village in central Bangladesh (MBG4,11,13,14) and more distantly with 2 performing M. mulatta (origin unknown) sampled near her village (MBG7 and MBG8). The virus sequence of BGH4 is equidistant from that of MBG7 and MBG8 and from that obtained from an M. mulatta (SFVmulO of unknown origin) housed at the Oregon National Regional Primate Center. SFV pol and gag sequences from HAD3 (from central Bali) clustered most closely with SFV from AK M. fascicularis at the Bali temple site where HAD3 worked, as did HAD38 pol sequences. In contrast, the virus sequences from these 2 humans were more distantly related to those from the UB animals, which were also M. fascicularis but from another temple site in Bali (≈15 km away). The SFV sequences from HAD3 and HAD38 were even less similar to SFV isolated from M. fascicularis from Singapore (SM isolates).
These data suggest that SFV sequences are stable in nonhuman primates and can be used for several macaque species to mark an individual’s geographic origin. Correlation between the SFV sequences isolated from humans and those from the corresponding nonhuman primate populations with which they reported contact was excellent.
We found prevalence of SFV infection in the heterogeneous populations studied to be 2.6%. In contrast with previous studies of persons who had occupational exposure to nonhuman primates, the exposure of some of the SFV-infected persons in our study was only through their normal daily routines. Previous research on nonhuman primate–human interaction in South and Southeast Asia describes interspecies contact as a frequent phenomenon in this part of the world (29,30). Our study takes this line of inquiry a step further, indicating that interspecies contact leads to nonhuman primate–to-human transmission of SFV in a variety of contexts, in several countries, and from multiple macaque species (Figure 3).
Bites from nonhuman primates are thought to be the most likely route of SFV transmission because viral RNA is found at high concentrations in the oral mucosa and saliva of infected animals (23). Indeed, 6 of the 8 SFV-infected persons reported having been bitten by a macaque at least 1 time. Although bites were reported by most SFV-positive persons, 2 denied having ever been bitten by a nonhuman primate. Possible explanations are that persons living in a community with a constant presence of nonhuman primates may not regard contacts, even scratches and bites, as notable events or, alternatively, that SFV is transmissible by contact other than bites, such as scratches or contact with nonhuman primate body fluids through breaks in the skin.
Other studies have shown SFV sequences to be highly stable (12,31). Switzer et al. (19) previously reported that they could determine the source chimpanzee of SFV infections in zoo workers by using phylogenetic analyses. We expanded those data to link SFV infections in populations exposed to free-ranging nonhuman primates to animals from their village and, in 1 instance, to differentiate native and introduced macaques solely by their SFV sequences (Figure 2, panel A). The 3 persons from whom SFV sequences were obtained each interacted with a single species of macaque; we did not detect any recombinant viruses, which are more likely to be encountered in persons who come into contact with multiple nonhuman primate species.
A recent review article recapitulates arguments that 2 factors influence the likelihood that disease can be transmitted from an animal reservoir to humans (32). First, phylogenetic relatedness suggests that the more closely a species is related to Homo sapiens, the more likely it is that transmission to humans can occur. The second factor is interspecies contact, which can be conceived as having 2 dimensions: the duration of contact and the intensity of contact. In general, contacts such as bites, scratches, or mucosal splashing with body fluids have the highest potential for transmitting infectious agents. In this light, the human–nonhuman primate interface in South and Southeast Asia ranks among the most likely contexts for zoonotic transmission.
In South and Southeast Asia, macaque monkeys and humans exhibit higher rates of sympatry than any other human–nonhuman primate overlap, owing in part to the major roles that nonhuman primates play in Hindu and Buddhist mythology and folklore. As a result, nonhuman primates are woven culturally and physically into the fabric of everyday life for millions of people. At least 68 temples throughout Thailand are home to populations of free-ranging nonhuman primates (5). Villagers in the town of Lopburi contend on a daily basis with >1,000 long-tailed macaques who spill out from the Pra Prang Sam Yot temple. These monkeys and the annual Monkey Buffet Festival (at which a buffet of fruits and vegetables is provided for all of the province’s monkeys) are a major tourist attraction. New Delhi, one of the most populous cities in the world, is also home to ≈5,000 free-ranging rhesus macaques. Interspecies contact leading to nonhuman primate bites is a familiar and increasing phenomenon in communities such as these and for their international tourists (33,34). The 5 major monkey temples in Bali collectively attract up to 700,000 visitors a year, most of whom feed monkeys and thousands of whom are bitten and/or scratched. Engel et al. recently published an analysis that used mathematical modeling to predict the likelihood of a visitor to a Balinese monkey temple becoming infected with SFV (29); this model predicted infection for ≈6 of every 1,000 visitors.
Two trends promise to increase human–nonhuman primate contact in South and Southeast Asia: nonhuman primate ecologic resilience and human alterations of the landscape. Because of the first trend, ecologic resilience and high birth rates, many populations of protected (sometimes fed as well) nonhuman primates are increasing rapidly. For example, during the 1990s, population levels of the 3 species of macaques in the Kowloon Hills of Hong Kong increased 100% (35). A second trend is habitat loss leading to increased concentrations of nonhuman primate populations in areas more densely populated by humans. In the northern Indian state of Himachal Pradesh, 86% (258,000) of rhesus macaques now inhabit urban areas as a result of habitat loss (11). This trend of increased urbanization of nonhuman primates is mirrored throughout Asia (36,37). In contrast, bushmeat hunting, the most common human–nonhuman primate interaction in Africa, is likely to decrease interspecies contact over time, as wild nonhuman primate populations continue to dwindle. These demographic facts lead us to echo previous calls for a global surveillance network to monitor the emergence of zoonotic disease, with the crucial caveat that such a network focus on areas of highest interspecies contact.
Our data suggest that the number of persons at risk for infection with SFV is much larger in South and Southeast Asia than elsewhere. This finding presents both a challenge and an opportunity for future research. The challenge is to find infected persons and follow the course of infection in addition to taking action to prevent future transmission. The opportunity lies in assembling a large cohort of infected persons, which will enable the use of epidemiologic techniques to learn about the natural course of SFV infection in humans.
Dr Jones-Engel is a senior research scientist in the Division of International Programs, University of Washington, National Primate Research Center. Her research focuses on bidirectional pathogen transmission between humans and primates in Asia and its implications for public health and primate conservation.
Acknowledgments
We thank the National Research Council of Thailand as well as Suvichai Rojanasathien, Boonraksar Soonthorntham, and Narit Sitasuwan (Thailand); the Pusat Studi Satwa Primata and Lembaga Ilmu Pengetahuan (Indonesia); the Federation of Swoyambhu Management and Conservation Committee (Nepal); and Khandaker Rahman (Bangladesh) for their support. We also thank N. Lerche, J. Yee, S. Begum, M. Aziz, M. Hasan, J. Heidrich, G. Kelemen, D. Cohn, C. Somgird, T. Sutthipat, R. Gharti, D. R. Shakya, G. Emel, P. Padungtod, P. Temeesiw, P. Daram, E. McArthur, C. Howard, and Hanna and Leah Engel for excellent technical and field assistance. Finally we thank Craig Bierle, Elizabeth Greene, and Ananias Escalante for their assistance with sequence analyses.
Work of M.L.L. was supported by National Institutes of Health (NIH)–National Cancer Institute grant R01CA81297 and Institutional Pilot Funds from the Fred Hutchinson Cancer Research Center. L.J.-E., G.A.E., and R.G. were supported by the Royalty Research Fund from the University of Washington, Puget Sound Partners for Global Health, Defense Advanced Research Projects Agency grant N66001-02-C-8072, and NIH-National Center for Research Resources grant P51 RR000166. Work of J.S.A was supported by NIH grant P51 RR013986-06. K.A.S. was partially supported by NIH training grant T32 CA09229.
References
- Chapman C, Gillespie T, Goldberg T. Primates and the ecology of their infectious diseases: how will anthropogenic change affect host-parasite interactions?Evol Anthropol. 2005;14:134–44. DOIGoogle Scholar
- Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife.Acta Trop. 2001;78:103–16. DOIPubMedGoogle Scholar
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities.Nature. 2000;403:853–8. DOIPubMedGoogle Scholar
- Chomel BB, Belotto A, Meslin FX. Wildlife, exotic pets, and emerging zoonoses.Emerg Infect Dis. 2007;13:6–11.PubMedGoogle Scholar
- Aggimarangsee N. Survey for semi-tame colonies of macaques in Thailand.Natural History Bulletin of the Siam Society.1992;40:103–66.
- Schillaci MA, Jones-Engel L, Engel GA, Paramastri Y, Iskandar E, Wilson B, Prevalence of enzootic simian viruses among urban performance monkeys in Indonesia.Trop Med Int Health. 2005;10:1305–14.PubMedGoogle Scholar
- Southwick CH, Siddiqi MF. Population status of nonhuman primates in Asia, with emphasis on rhesus macaques in India.Am J Primatol. 1994;34:51–9. DOIGoogle Scholar
- Wolcott LT. Hanuman: power-dispensing monkey in North Indian folk religion.J Asian Stud. 1978;37:653–61. DOIGoogle Scholar
- Jones-Engel L, Engel GA, Heidrich J, Chalise M, Poudel N, Viscidi R, Temple monkeys and health implications of commensalism, Kathmandu, Nepal.Emerg Infect Dis. 2006;12:900–6.PubMedGoogle Scholar
- Schillaci M, Jones-Engel L, Engel G, Fuentes A. Characterizing the threat to the blood supply associated with nonoccupational exposure to emerging simian retroviruses.Transfusion. 2008;48:398–401.PubMedGoogle Scholar
- Southwick C, Malik I, Siddiqi MF. Rhesus commensalism in India: problems and prospects. In: Patterson J, Wallis J, editors. Commensalism and conflict: human-primate interface. Norman (OK): American Society of Primatologists; 2005. p. 240–57.
- Switzer WM, Salemi M, Shanmugam V, Gao F, Cong ME, Kuiken C, Ancient co-speciation of simian foamy viruses and primates.Nature. 2005;434:376–80. DOIPubMedGoogle Scholar
- Linial ML. Foamy viruses. In: Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. p. 2245–63.
- Calattini S, Wanert F, Thierry B, Schmitt C, Bassot S, Saib A, Modes of transmission and genetic diversity of foamy viruses in a Macaca tonkeana colony.Retrovirology. 2006;3:23. DOIPubMedGoogle Scholar
- Jones-Engel L, Steinkraus KA, Murray SM, Engel GA, Grant R, Aggimarangsee N, Sensitive assays for simian foamy viruses reveal a high prevalence of infection in commensal, free-ranging Asian monkeys.J Virol. 2007;81:7330–7. DOIPubMedGoogle Scholar
- Calattini S, Betsem EBA, Froment A, Mauclere P, Tortevoye P, Schmitt C, Simian foamy virus transmission from apes to humans, rural Cameroon.Emerg Infect Dis. 2007;13:1314–20.PubMedGoogle Scholar
- Jones-Engel L, Engel GA, Schillaci MA, Rompis A, Putra A, Suaryana KG, Primate-to-human retroviral transmission in Asia.Emerg Infect Dis. 2005;11:1028–35.PubMedGoogle Scholar
- Sandstrom PA, Phan KO, Switzer WM, Fredeking T, Chapman L, Heneine W, Simian foamy virus infection among zoo keepers.Lancet. 2000;355:551–2. DOIPubMedGoogle Scholar
- Switzer WM, Bhullar V, Shanmugam V, Cong ME, Parekh B, Lerche NW, Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates.J Virol. 2004;78:2780–9. DOIPubMedGoogle Scholar
- Wolfe ND, Switzer WM, Carr JK, Bhullar VB, Shanmugam V, Tamoufe U, Naturally acquired simian retrovirus infections in central African hunters.Lancet. 2004;363:932–7. DOIPubMedGoogle Scholar
- Boneva RS, Switzer WM, Spira TJ, Bhullar VB, Shanmugam V, Cong ME, Clinical and virological characterization of persistent human infection with simian foamy viruses.AIDS Res Hum Retroviruses. 2007;23:1330–7. DOIPubMedGoogle Scholar
- Kuller L, Watanabe R, Anderson D, Grant R. Development of a whole-virus multiplex flow cytometric assay for antibody screening of a specific pathogen-free primate colony.Diagn Microbiol Infect Dis. 2005;53:185–93. DOIPubMedGoogle Scholar
- Murray SM, Picker LJ, Axthelm MK, Linial ML. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression.J Virol. 2006;80:663–70. DOIPubMedGoogle Scholar
- Schweizer M, Neumann-Haefelin D. Phylogenetic analysis of primate foamy viruses by comparison of pol sequences.Virology. 1995;207:577–82. DOIPubMedGoogle Scholar
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees.Mol Biol Evol. 1987;4:406–25.PubMedGoogle Scholar
- Tajima F, Nei M. Estimation of evolutionary distance between nucleotide sequences.Mol Biol Evol. 1984;1:269–85.PubMedGoogle Scholar
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0.Mol Biol Evol. 2007;24:1596–9. DOIPubMedGoogle Scholar
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees.Bioinformatics. 2001;17:754–5. DOIPubMedGoogle Scholar
- Engel G, Hungerford LL, Jones-Engel L, Travis D, Eberle R, Fuentes A, Risk assessment: a model for predicting cross-species transmission of simian foamy virus from macaques (M. fascicularis) to humans at a monkey temple in Bali, Indonesia.Am J Primatol. 2006;68:934–48. DOIPubMedGoogle Scholar
- Fuentes A. Human culture and monkey behavior: assessing the contexts of potential pathogen transmission between macaques and humans.Am J Primatol. 2006;68:880–96. DOIPubMedGoogle Scholar
- Schweizer M, Schleer H, Pietrek M, Liegibel J, Falcone V, Neumann-Haefelin D. Genetic stability of foamy viruses: long-term study in an African green monkey population.J Virol. 1999;73:9256–65.PubMedGoogle Scholar
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases.Nature. 2007;447:279–83. DOIPubMedGoogle Scholar
- Gautret P, Schwartz E, Shaw M, Soula G, Gazin P, Delmont J, Animal-associated injuries and related diseases among returned travellers: a review of the GeoSentinel Surveillance Network.Vaccine. 2007;25:2656–63. DOIPubMedGoogle Scholar
- Singla SL, Kaur M, Lal S. Monkey bites: a public health problem in urban setting.Indian J Public Health. 1997;41:3–5, 24.PubMedGoogle Scholar
- Wong CL, Ni IH. Population dynamics of the feral macaques in the Kowloon Hills of Hong Kong.Am J Primatol. 2000;50:53–66. DOIPubMedGoogle Scholar
- Watanabe K, Muroyama Y. Recent expansion of the range of Japanese macaques and associated management problems. In: Patterson J, Wallis J, editors. Commensalism and conflict: the primate-human interface. Norman (OK): American Society of Primatologists; 2005. p. 400–20.
- Zhao QK. Tibetan macaques, visitors and local people at Mt. Emei: problems and countermeasures. In: Patterson J, Wallis J, editors. Commensalism and conflict: the primate-human interface. Norman (OK): American Society of Primatologists; 2005. p. 376–99.
Figures
Tables
Cite This ArticleTable of Contents – Volume 14, Number 8—August 2008
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Lisa Jones-Engel, Division of International Programs, National Primate Research Center, University of Washington, 1705 Pacific NE, HSB I-039, Seattle, WA 98195, USA;
Top