Volume 15, Number 11—November 2009
Dispatch
Hemorrhagic Fever with Renal Syndrome in 4 US Soldiers, South Korea, 2005
Cite This Article
Citation for Media
Abstract
Four US soldiers acquired hemorrhagic fever with renal syndrome while training near the Demilitarized Zone, South Korea, in 2005. Hantaan virus sequences were amplified by reverse transcription–PCR from patient serum samples and from lung tissues of striped field mice (Apodemus agrarius) captured at training sites. Epidemiologic investigations specified the ecology of possible sites of patient infection.
Hantaan virus (HTNV), the etiologic agent for hemorrhagic fever with renal syndrome (HFRS), accounts for ≈70% of all HFRS cases in South Korea and is the most severe of the 4 rodent-borne hantaviruses (Seoul virus, Soochong virus, and Muju virus) found there (1–3). Recently, a shrew-borne hantavirus, Imjin virus, was isolated from Ussuri white toothed shrews (Crocidura lasiura) captured near the Imjin River in South Korea (4). The reservoir host of HTNV, the striped field mouse (Apodemus agrarius), is the most abundant field rodent found in South Korea. We conducted an epidemiologic investigation for rodents at 6 training sites near the Demilitarized Zone after 4 US soldiers acquired HFRS in 2005, because no evidence of rodent activity was found where the soldiers worked or resided at their base camp (Camps Hovey and Casey).
On October 27, 2005, a US soldier (patient 1) assigned to Camp Hovey, Dongducheon, exhibited signs and symptoms of HFRS (Table 1). The patient was transferred to the Brian Allgood Army Community Hospital (BAACH), Yongsan Army Garrison, Seoul, South Korea, and on October 28, 2005, was confirmed to be seropositive for hantavirus infection by ELISA. A medical advisor initially suspected that the patient may have acquired the infection while sweeping out a dusty storage area at Camp Hovey, where he resided, ≈3 days before the onset of symptoms. A survey of the suspected storage room did not uncover any signs of rodent activity. These data along with the known incubation period of hantaviruses (4–>50 days) prompted a further search for the actual site of transmission.
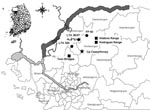
A blood sample from patient 1, tested by both the indirect immunofluorescent antibody (IFA) technique and reverse transcription–PCR (RT-PCR), confirmed that the patient was infected with HTNV. An epidemiologic survey was conducted, and results showed that the patient had trained at 4 US- and South Korea–operated training sites from 26 to 35 days before the onset of symptoms. The patient had first trained at local training area (LTA) 320, then at LTA 36/37, and finally at firing point (FP)-60 (Figure 1). The training consisted of setting up firing positions, establishing a cantonment site, and performing other training activities for 5 days before moving on to FP-60, where troops conducted firing exercises from September 25–29, 2005, before returning to Camp Hovey.
On November 8–9, 2005, two soldiers (patients 2 and 3) from Camp Casey, Dongducheon, had signs and symptoms of HFRS and so were sent to the BAACH where they received a diagnosis of HFRS (Table 1). Patient 4 from the same unit sought treatment at the Camp Casey Troop Medical Clinic on November 13 with low-grade fever, decreased appetite, abdominal pain, chills, low back pain, nausea, and vomiting, and was provided fluids and assigned to quarters (home bed rest). On November 14, laboratory results indicated a nonspecific proteinuria characteristic of HFRS infections, and the patient was transported to the BAACH, where HFRS was diagnosed. Blood samples from all 3 patients were positive for HTNV by IFA and RT-PCR. All 3 patients had trained at Twin Bridges Training Area (TBTA) with potential incubation periods that ranged from 16 to 35 days, and 2 (patients 2 and 4) had trained together at Rodriguez and Watkins Ranges 3 days before conducting a tactical move to TBTA (Table 1; Figure 1). Patients 2 and 4 conducted training at TBTA-North (N) and TBTA-South (S), and patient 3 only conducted training at TBTA-N.
Small mammal trapping was conducted at US and South Korea–operated training sites where the HFRS patients had previously trained within 60 days. Preseasonal (September) A. agrarius mice trapping rates were relatively low at both FP-60 (11.4%) and Rodriguez Range (7.6%), and although postseasonal trapping rates were high at FP-60 (42.3%), they remained relatively low at Rodriguez Range (12.1%) (Table 2). During the fall, hantavirus seropositive rates were high at FP-60 (20.0%) and Rodriguez Range (31.3%). During the winter, seropositive rates increased at FP-60 (25.8%), but decreased at Rodriguez Range (15.7%). During the winter, seropositive rates at TBTA-N (26.3%) and TBTA-S (37.8%) were high, but were relatively low at other training sites (LTA320/36/37) surveyed.
Blood samples from each of the 4 patients and lung tissues of seropositive rodents were assayed by RT-PCR. RNA extracted by using RNA-Bee isolation kit (TEL-TEST Inc., Friendswood, TX, USA) was reverse transcribed by using the superscript II RNase H-reverse transcriptase kit (GIBCO-BRL, Gaithersburg, MD, USA). Primers (outer primer set, 5′-TGGGCTGCAAGTGC-3′, 5′-ACATGC TGTACAGCCTGTGCC-3′; inner primer set, 5′-TGGGCTGCAAGTGCATCAGAG-3′, 5′-ATGGATTACAACCCCAGCTCG-3′) amplified a 373-nt region of the hantavirus G2-encoding medium (M) segment (1,5,6). Amplified products were fractionated according to size by electrophoresis on 1.5% agarose gels containing ethidium bromide (0.5 mg/mL). DNA sequencing was performed in both directions, by using the dye primer cycle sequencing ready reaction kit (Applied Biosystems, Foster City, CA, USA) on an automated sequencer (Model 3730XL; Applied Biosystems).
Phylogenetic analysis, both neighbor-joining and maximum-parsimony methods, based on the 320-nt region of the G2 glycoprotein–encoding M segment of the 4 HFRS patients, and HTNV sequences amplified from A. agrarius mice captured at FP-10, FP-60, TBTA-N, and TBTA-S demonstrated that the HTNV sequence amplified from patient 1 was identical to sequences from the A. agrarius HTNV strain (04–1325) at FP-60. The analyses also demonstrated that the HTNV sequences from patients 2 and 4 were identical to A. agrarius HTNV sequence (05–1437) at TBTA-S, and the HTNV sequence from patient 3 was identical to A. agrarius HTNV sequences (07–196 and 05–1465) at TBTA-N. These data demonstrate the most likely site of infection for the 4 HFRS patients (Figure 2).
The relationships between rodent density, the proportion of hantavirus-seropositive rodents, and incidence of human infection are complex and poorly understood (7,8). Previous literature indicates that a prevalence of hantavirus seropositivity >20% among A. agrarius mice greatly increased the risk for transmission (9–11). Although monitoring rodent populations may provide some warning, the most effective means of controlling hantavirus infections is limiting human contact with rodents and the inhalation of dust with virus-laden rodent excreta (12).
The large patches of tall dense grasses narrowly separated by barren ground where artillery firing is conducted at FP-60 provide harborage for A. agrarius mice and, during the winter trapping period, yielded ≈20% capture rates (10). Eliminating these large grassy islands, and thus the rodents that inhabit these areas, and cutting the tall grasses and scrub vegetation to <10 cm along the training site perimeter would decrease hantavirus infection risks by reducing the rodent populations. Efforts to mitigate disease risks through modernization of training sites, cutting of vegetation that increases predation of small mammals, and habitat reduction within 50 m of military operations, where possible, are being instituted at selected US-operated training sites as a result of consolidation and modernization of large, multipurpose range complexes.
Previously, phylogenetic analysis of a 324-nt region of the G2 glycoprotein-encoding M genomic segment has been shown to be representative of the entire M segment (6). This region may be useful for classifying newly identified hantaviruses (when cross-neutralization data are unavailable) or for further analysis of molecular phylogeny of hantaviruses spatially. The genome of HTNV sequences obtained from patient 1 was identical to a viral sequence from an A. agrarius field mouse captured at FP-60 and those from patients 2 and 4 were identical to 3 viral sequences from A. agrarius at TBTA-South, and patient 3 was identical to 11 viral sequences from A. agrarius field mice at TBTA-North. These data showed the epidemiologic link between US soldier patients and rodent hosts at the training sites near the Demilitarized Zone in South Korea.
Dr Jin-Won Song is a professor of microbiology at Korea University. He has had a long-term interest in the global epizootiology and epidemiology of hantaviruses.
Acknowledgments
We thank Brian Allgood (deceased), Martha Sanders, and Hee-Choon Lee for their support in pursuing the epidemiologic investigations leading to identification of sites of HFRS transmission. We thank Suk Hee Yi for analysis of data and GIS mapping. We also thank Jiun Yoon, Amy Nguyen, Min Ro, and Rex Bergren for their support.
Funding for portions of this work was provided by the Armed Forces Health Surveillance Center, Global Emerging Infections Surveillance and Response System, Silver Spring, Maryland, USA, and the National Center for Medical Intelligence Center, Fort Detrick, Maryland, USA.
References
- Song J-W, Baek LJ, Kim SH, Kho EY, Kim JH, Yanagihara R, Genetic diversity of Apodemus agrarius-borne Hantaan virus in Korea. Virus Genes. 2000;21:227–32. DOIPubMedGoogle Scholar
- Baek LJ, Kariwa H, Lokugamage K, Yoshimatsu K, Arikawa J, Takashima I, Soochong virus: an antigenically and genetically distinct hantavirus isolated from Apodemus peninsulae in Korea. J Med Virol. 2006;78:290–7. DOIPubMedGoogle Scholar
- Song K-J, Baek LJ, Moon S, Ha SJ, Kim SH, Park KS, Muju virus, a novel hantavirus harboured by the arvicolid rodent Myodes regulus in Korea. J Gen Virol. 2007;88:3121–9. DOIPubMedGoogle Scholar
- Song J-W, Kang HJ, Gu SH, Moon SS, Bennett SN, Song KJ, Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura). J Virol. 2009;83:6184–91. DOIPubMedGoogle Scholar
- Xiao SY, Chu YK, Knauert FK, Lofts R, Dalrymple JM, LeDuc JW. Comparison of hantavirus isolates using a genus-reactive primer pair polymerase chain reaction. J Gen Virol. 1992;73:567–73. DOIPubMedGoogle Scholar
- Xiao SY, LeDuc JW, Chu YK, Schmaljohn CS. Phylogenetic analyses of virus isolates in the genus Hantavirus, family Bunyaviridae. Virology. 1994;198:205–17. DOIPubMedGoogle Scholar
- Niklasson B, Hornfeldt B, Lundkvist A, Bjorsten S, LeDuc J. Temporal dynamics of Puumala virus antibody prevalence in voles and of nephropathia epidemica incidence in humans. Am J Trop Med Hyg. 1995;53:134–40.PubMedGoogle Scholar
- Olsson GE, Dalerum F, Hornfeldt B, Elgh F, Palo TR, Juto P, Human hantavirus infections, Sweden. Emerg Infect Dis. 2003;9:1395–401.PubMedGoogle Scholar
- Hong HK, Lee UY. Studies on the biology of Apodemus agrarius in Korea. Annual Report Collection of Incheon University. 1984;6:417–39.
- O’Guinn ML, Klein TA, Lee JS, Kim HC, Baek LJ, Chong ST, Ecological surveillance of small mammals at firing points 10 and 60, Gyeonggi Province, Republic of Korea, 2001–2005. J Vector Ecol. 2008;33:370–84. DOIPubMedGoogle Scholar
- Lee HW, Baek LJ, Doo CD. The study on breeding season of Apodemus agrarius, the natural host of Korean hemorrhagic fever [in Korean]. J. Korean Soc Virol. 1981;11:1–5.
Figures
Tables
Cite This Article1Current affiliation: Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
2Current affiliation: University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Table of Contents – Volume 15, Number 11—November 2009
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Jin-Won Song, Department of Microbiology, College of Medicine, Korea University, 126-1, 5Ka, Anam-Dong, Sungbuk-Gu, Seoul 136-705, South Korea
Top