Volume 15, Number 11—November 2009
CME ACTIVITY - Research
Multicenter GeoSentinel Analysis of Rickettsial Diseases in International Travelers, 1996–2008
Cite This Article
Citation for Media
Introduction

MedscapeCME is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit. This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of MedscapeCME and Emerging Infectious Diseases. MedscapeCME is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. MedscapeCME designates this educational activity for a maximum of 0.75 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.com/cme/eid; (4) view/print certificate.
Learning Objectives
Upon completion of this activity, participants will be able to:
- Describe the most common rickettsial diseases in returning international travelers between 1996 and 2008.
- Identify risk factors associated with higher likelihood of rickettsial disease among returning international travelers.
- Describe the most common treatment for rickettsial diseases.
CME Editor
Nancy Farm Männikkö, PhD, Technical Writer-Editor, Emerging Infectious Diseases. Disclosure: Nancy Farm Männikkö, PhD, has disclosed no relevant financial relationships.
CME Author
Désirée Lie, MD, MSEd, Clinical Professor, Family Medicine, University of California, Orange; Director, Division of Faculty Development, UCI Medical Center, Orange, California. Disclosure: Désirée Lie, MD, MSEd, has disclosed no relevant financial relationships.
Authors
Disclosures: Mogens Jensenius, MD, PhD; Xiaohong Davis, PhD; Frank von Sonnenburg, MD; Eli Schwartz, MD; Jay S. Keystone, MD, MSc, FRCPC; Karin Leder, FRACP,; Rogelio Lopéz-Véléz, MD, PhD; Jakob P. Cramer, MD; Lin Chen, MD; and Philippe Parola, MD, have disclosed no relevant financial relationships. Eric Caumes, MD, has disclosed the following relevant financial relationships: served an as advisor or consultant for Novartis Pharmaceuticals Corporation; served as a speaker or a member of a speakers bureau for Wyeth France.
We investigated epidemiologic and clinical aspects of rickettsial diseases in 280 international travelers reported to the GeoSentinel surveillance Network during 1996–2008. Of these 280 travelers, 231 (82.5%) had spotted fever (SFG) rickettsiosis, 16 (5.7%) scrub typhus, 11 (3.9%) Q fever, 10 (3.6%) typhus group (TG) rickettsiosis, 7 (2.5%) bartonellosis, 4 (1.4%) indeterminable SFG/TG rickettsiosis, and 1 (0.4%) human granulocytic anaplasmosis. One hundred ninety-seven (87.6%) SFG rickettsiosis cases were acquired in sub-Saharan Africa and were associated with higher age, male gender, travel to southern Africa, late summer season travel, and travel for tourism. More than 90% of patients with rickettsial disease were treated with doxycycline, 43 (15.4%) were hospitalized, and 4 had a complicated course, including 1 fatal case of scrub typhus encephalitis acquired in Thailand.
Rickettsial diseases are acute and potentially severe zoonotic infections caused by obligate intracellular, gram-negative bacteria belonging to the order Rickettsiales. The taxonomy of Rickettsiales is complex and continues to be updated, but currently the agents of rickettsial diseases are classified as belonging to 4 distinct genera: Rickettsia (including 2 biogroups: spotted fever group [SFG] rickettsiae with >10 species and typhus group [TG] rickettsiae with 2 species), Orientia (Orientia tsutsugamushi, the agent of scrub typhus), Ehrlichia (Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis), and Anaplasma (Anaplasma phagocytophilium, the agent of human granulocytic anaplasmosis). Diseases caused by Rickettsia and Orientia species are often collectively referred to as rickettsioses. Coxiella burnetii, the agent of Q fever, and Bartonella spp. were recently removed from the order Rickettsiales, but Q fever and bartonelloses are still frequently categorized as rickettsial diseases (1).
Rickettsial diseases are increasingly being recognized among international travelers (2). A recent study of ≈7,000 returnees with fever as a chief reason to seek medical care suggests that 2% of imported fevers are caused by rickettsioses and that 20% of these patients are hospitalized (3). Most cases are acquired in sub-Saharan Africa, where SFG rickettsioses are second only to malaria as the most commonly diagnosed diseases in returnees with systemic febrile illness (4). With few exceptions, however, our knowledge of the incidence rates, associated factors, signs, symptoms, and outcome of rickettsial diseases in travelers is rudimentary and mostly based on smaller case series. We report all cases of rickettsial diseases in returned travelers reported to the GeoSentinel Surveillance Network from June 1996 through December 2008.
Data Source
GeoSentinel is a global network aimed at surveying ill international travelers and was established in 1995 through the International Society of Travel Medicine and the Centers for Disease Control and Prevention. The 41 current GeoSentinel sites contribute clinician-based, anonymous information on all ill travelers seen that is entered in a structured query language database at a central data center. Data collected include demographic information, recent travel history, reason for travel, outpatient or inpatient status, whether the patient was seen during or after travel, whether the patient traveled independently versus organized travel (i.e., travel with a tour group), whether the patient had pretravel encounter with a healthcare provider, patient symptoms, and diagnosis. All GeoSentinel sites use the best reference diagnostic methods available in the country where the site is located. The diagnosis, which may be reported as confirmed, probable, or suspected, is chosen from a standardized list of >500 possible etiologic and syndromic diagnoses, 11 of which refer to rickettsial diseases. Patients may receive several diagnoses, some of which describe well-known complications of rickettsial diseases, e.g., acute renal failure, acute encephalitis, acute respiratory distress syndrome, and death (3,4). The GeoSentinel questionnaire does not contain queries about case management, but in December 2008 all sites were requested to estimate the percentage of their patients with rickettsial diseases who received treatment with antirickettsial drugs (e.g., doxycycline) during the study period.
Inclusion Criteria and Definitions
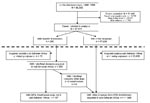
Data entered into the GeoSentinel database from June 1996 through December 2008 were reviewed. Only travelers seen with confirmed or probable diagnoses were included in the analysis. Cases of rickettsial diseases were defined according to the criteria mentioned below. A subanalysis, comparing SFG rickettsioses with other diseases in ill returnees from sub-Saharan Africa, was also performed (Figure 1).
A microbiologic diagnosis of recent rickettsial disease was in most cases based on immunofluorescence antibody (IFA) tests and, occasionally, on PCR and Western blot. Diagnostic criteria of recent infection by IFA tests were either a 4-fold increase of immunoglobulin (Ig) G or IgM titers in paired serum samples drawn >10 days apart, or elevated IgG and/or IgM titers in single samples consistent with recent infection as interpreted by the local microbiology laboratory. Because IFA tests cannot distinguish between species within the same Rickettsia biogroup, diseases caused by Rickettsia species were dichotomized as SFG rickettsiosis and TG rickettsiosis.
We included all cases of confirmed rickettsial disease defined as a traveler with pertinent travel history and clinical signs, and microbiologic test results supporting recent infection. For SFG rickettsiosis and scrub typhus we also included probable cases defined as a traveler with pertinent travel history and clinical signs including >1 inoculation eschars, and inconclusive or unavailable microbiologic test results. Cases were categorized in 9 disease groups: SFG rickettsiosis, TG rickettsiosis, indeterminate SFG/TG rickettsiosis (defined as cases in whom neither clinical signs nor microbiologic tests could distinguish between SFG and TG rickettsiosis), scrub typhus, ehrlichiosis, anaplasmosis, acute and chronic Q fever (based on the presence of antibodies to the 2 distinct antigenic phases of C. burnetii by IFA test [5]), and bartonellosis. Countries were grouped according to the United Nations region system, and for sub-Saharan Africa also in subregions; for clarification, the southern Africa subregion included Botswana, Lesotho, Namibia, South Africa, and Swaziland. Classification of reason for travel related to current illness was grouped into 4 categories: tourism, business, visiting friends and relatives, and other reason, which included studies or military, missionary, or foreign aid deployment.
Statistical Analysis
All data were analyzed using SAS software, version 9 (SAS Institute, Cary, NC, USA). Comparison of travelers to sub-Saharan Africa with SFG rickettsioses with all other ill travelers to the same destination used the Student t test for continuous variables and Cochran-Mantel-Haenszel statistic for binary variables. The association between possible risk factors and a diagnosis of SFG rickettsioses acquired in sub-Saharan Africa was measured with odds ratios and 95% confidence intervals. Variables included in the univariate analysis were mean age, male gender, travel to southern Africa, travel from March through May, travel >1 week, no pretravel clinic visit, independent travel, and tourism as reason for travel. Variables with a p value <0.05 in the final multivariate logistic regression model were considered significant.
Proportionate morbidity for SFG rickettsiosis acquired in sub-Saharan Africa was defined as the number of cases as a proportion of all ill returned travelers from the region. Analysis of case reports over time was based on monthly proportionate morbidity, i.e., the number of patients with SFG rickettsiosis as a proportion of the number of all ill returned travelers attending any GeoSentinel site in that month. The monthly seasonality was based on aggregate data for that month over all years. The annual proportionate morbidity for SFG rickettsiosis acquired in southern Africa from 1999 through 2008 was estimated in a similar way based on relevant cases for each year. The Cochran-Armitage test was used for testing trend over years. This statistic tests for trend in binomial proportions across levels of a single factor or covariate and is appropriate for a contingency table where the response variable has 2 levels and the explanatory variable is ordinal. A 1-sided p value <0.05 would indicate a decreasing (with a negative statistic) or increasing (with a positive statistic) trend.
We identified 99,355 travelers who sought medical care at a GeoSentinel site during June 1996–December 2008. Among the 47,915 case-patients who met the study inclusion criteria, 280 (0.6%) had a diagnosis of rickettsial disease (Figure 1); among those having fever, the proportion was 211/13,763 (1.5%). Of travelers with rickettsial disease 231 (82.5%) had SFG rickettsioses, 16 (5.7%) scrub typhus, 11 (3.9%) acute Q fever, 10 (3.6%) TG rickettsioses, 7 (2.5%) bartonelloses, 4 (1.4%) indeterminate TG/SFG rickettsioses, and 1 (0.4%) human granulocytic anaplasmosis; there were no cases of chronic Q fever and ehrlichiosis. Cases were reported from 32 of the 41 active GeoSentinel sites: a total of 154 (54.9%) were reported from sites in Europe, 77 (27.5%) from North America, 17 (6.0%) from New Zealand/Australia, and 32 (11.6%) from Asia, including the Middle East. Most cases were associated with travel to sub-Saharan Africa (75.1%) (Table 1). A pretravel encounter with a healthcare provider was reported in 157 cases (58.4%). At least 90% of cases of rickettsial diseases were estimated to have been treated with antirickettsial drugs (doxycycline in most cases, occasionally a flouroquinolone) at the reporting sites during the study period.
SFG Rickettsioses
Of the 231 cases of SFG rickettsioses (146 confirmed and 85 probable) a total of 136 (58.9%) were men; the mean age was 43.4 years (median 45 years, range 33–53 years). Tourists comprised 182 (78.8%), 28 (12.1%) had traveled for business, 6 (2.6%) were visiting friends and relatives, and 14 (6.1%) had traveled for other reasons. One hundred ninety-seven (87.6%) case-patients were infected in sub-Saharan Africa; South Africa (n = 135), Zimbabwe (n = 13), and Tanzania (n = 7) were the 3 most commonly reported countries of exposure (Table 1). The median time from travel to reporting to a GeoSentinel site was 8 days (range 4–12 days). Two case-patients, a 23-year-old French woman tourist to Mongolia in June 2008, and a 27-year-old Swiss woman student traveler to Corsica, France, in October 2008, were infected by Rickettsia slovaca confirmed by PCR and Western blot. There were no deaths among the travelers with SFG rickettsioses, but 22 (9.6%) were hospitalized, and multiorgan failure with acute respiratory distress syndrome developed in 2 Israeli men 47 and 73 years of age, respectively, after they traveled to India in October 2004.
Analysis of demographic and exposure variables for the 197 travelers to sub-Saharan Africa with SFG rickettsiosis compared with 11,690 travelers to the same region with other diagnoses is shown in Table 2. A multivariate logistic regression model was performed to analyze the effects on SFG rickettsiosis of risk factors including age, gender, travel to southern Africa, travel from March to May, travel duration longer than 7 days, no pretravel clinic visit, independent travel, and travel for tourism. Older age, male gender, travel to southern Africa, travel from March to May, and travel for tourism were found to be independently associated with SFG rickettsiosis. An analysis of monthly proportionate morbidity identified a peak of cases in March, April, and May (Figure 2). The proportionate morbidity of cases with SFG rickettsiosis in ill returnees from southern Africa from 1999 through 2008 was 139/1,017 (13.7%). The overall Cochran-Armitage trend test did not show statistically significant change (p = 0.877, by 2-sided trend test). As shown in Figure 3, the annual proportionate morbidity increased and decreased twice over the years and did not maintain a monotone increasing or decreasing pattern.
Other Rickettsial Diseases
The 10 travelers with TG rickettsiosis, of whom 6 were men, had a mean age of 28.0 years (median 25.5 years, range 20–50 years). Four case-patients were tourists, 2 were visiting friends and relatives, 1 was a business traveler, and 3 had traveled for other purposes. Southeast Asia, including 3 travelers infected in Indonesia, was the most common region of exposure (Table 1). The median time from travel to reporting a site was 6 days (range 3–14 days). Five patients were hospitalized and no complications were noted. One patient, a 23-year-old Japanese man visiting friends and relatives in Bali, Indonesia, had R. typhi infection confirmed by PCR.
Indeterminate SFG/TG rickettsiosis was diagnosed in 4 male tourists, 15, 50, 51, and 67 years of age, respectively. Two case-patients were infected in Southeast Asia (Table 1). No complications were recorded.
The 16 travelers with scrub typhus (5 confirmed and 11 probable), of whom 10 were men, had a mean age of 36.6 years (median 32.5 years; range 26–67 years). Nine patients were infected in Southeast Asia (including Thailand, n = 4) and 5 in south-central Asia (including India, n = 3). Twelve patients were tourists, 1 was visiting friends and relatives, and 3 had traveled for business. The median time from travel to reporting to a GeoSentinel site was 9 days (range 2–25 days). Six case-patients were hospitalized. Encephalitis developed in 2 Singaporean travelers: a 42-year-old man, a tourist infected in Thailand in December 2007, died, and a 51-year-old man infected in Taiwan while traveling on business in April 2008 survived.
An uncomplicated case of human granulocytic anaplasmosis was seen in August 2008 in a 32-year-old US woman tourist to the Netherlands. The diagnosis was based on detection of intracellular morulae.
The 11 travelers with acute Q fever, including 4 men, had a mean age of 53.0 years (median 59 years; range 20–64 years). Sub-Saharan Africa, including 2 case-patients infected in Tanzania, was the most common region of exposure (Table 1). Seven patients were tourists, 3 were business travelers, and 1 had traveled for other reasons. The median time from travel to reporting to a site was 21 days (range 5–52 days). Isolated fever (n = 7) and respiratory symptoms (n = 4) were the most common clinical manifestations. Four patients were hospitalized. All 11 patients with Q fever had uncomplicated clinical courses.
The 7 travelers with bartonellosis, including 3 males, had a mean age of 33.6 years (median 32 years, range 9–56 years). Three patients were infected in the Caribbean (Table 1). Four were tourists and 3 had traveled for other purposes. The median time from travel to reporting to a GeoSentinel site was 25 days (range 7–39 days). Bartonella henselae infection was diagnosed in all case-patients by serologic or immunohistochemical analysis. Six travelers had uncomplicated cat-scratch disease, and 1 had bacillary angiomatosis.
This study of 280 case-patients conducted over >12 years at 32 institutions on 5 continents represents the largest series of imported rickettsial diseases published to date. Noteworthy, SFG rickettsiosis was by far the most commonly diagnosed group of disease in our study. Numerous SFG rickettsioses occur throughout the world, the most important being Mediterranean spotted fever (with its variants Indian tick typhus, Astrakhan fever, and Israeli spotted fever) caused by R. conorii conorii, Rocky Mountain spotted fever caused by R. rickettsii, and African tick bite fever caused by R. africae (1). Some SFG rickettsioses may be accompanied by severe complications (6,7), as was exemplified by our 2 case-patients infected in India, possibly representing Indian tick typhus caused by R. conorii indica (8). Tick-borne lymphadenopathy (TIBOLA) caused by R. slovaca, a recently described entity associated with Dermacentor ticks and characterized by inoculation eschar, fatigue, and painful regional lymphadenitis (9), was diagnosed in 2 of our travelers. Being well documented across southern and central Europe, and especially so in children (10), our traveler infected in Mongolia represents the first documented case of TIBOLA acquired in Asia and illustrates how travel medicine may help identify new areas of endemicity for infectious diseases.
As shown here and by others (11), most cases of travel-associated SFG rickettsiosis are acquired in sub-Saharan Africa, and particularly in South Africa and neighboring countries. In the present series, as many as 13.7% of all healthcare-seeking returnees from southern Africa had a diagnosis of SFG rickettsiosis (Figure 3). Although several pathogenic SFG rickettsiae have been described in sub-Saharan Africa, including R. conorii, R. siberica, and R. aeschlimannii (12), 2 recent series evaluating 159 case-patients with species-specific tests, including PCR and Western blot, suggest that >99% of SFG rickettsioses diagnosed in international travelers to the region are caused by R. africae (13,14). In 1 of these reports, game hunting as reason for travel, travel to southern Africa, and travel during the summer season from November through April were found to be independent risk factors (14). If one assumes that most, if not all, of our 197 cases of SFG rickettsioses acquired in sub-Saharan Africa were de facto African tick bite fever, the present study suggests additional risk factors for this disease: male gender, higher age, travel during the late summer months of southern Africa (i.e., March–May; Figure 2), and travel for tourism. Although African tick bite fever is usually benign and self-limited, some patients may develop debilitating complications such as reactive arthritis, cranial and peripheral neuropathies, myocarditis, and neuropsychiatric symptoms, or experience a long-lasting convalescence, the latter phenomenon recently reported in elderly patients (15–17).
Our series comprises 10 cases of TG rickettsioses, all of which were considered to represent murine typhus caused by R. typhi, a bacteria transmitted from rodents to humans by rat fleas in many tropical and subtropical areas. Murine typhus is sometimes reported in returnees from the Mediterranean basin, Asia, and Africa; typical itineraries included travel to port cities or beach resorts (18,19). As in the present series, most infected travelers have mild disease with fever and constitutional symptoms, but complicated cases, including deaths, have been reported by others (20).
We also identified 16 cases of scrub typhus, a common disease in rural south and Southeast Asia and the Pacific. Before the present report, <30 cases of travel-associated scrub typhus had been published in the literature, and infection was predominantly acquired in Thailand and neighboring countries (21). The disease is transmitted by the bites of larval trombiculid mites (chiggers) that occur year round on grassy vegetation and is typically acquired by campers, trekkers, and visitors to rice paddies (2). Although most cases in travelers are mild and uncomplicated, scrub typhus may, as exemplified here, result in life-threatening encephalitis (22).
Human granulocytic anaplasmosis is a usually mild and nonspecific febrile illness commencing a few days after an Ixodes tick bite (23). It is endemic in North America and Europe with a geographic distribution that largely coincides with that of Lyme borreliosis. Human granulocytic anaplasmosis is rarely reported in international travelers but was recently diagnosed in an Austrian tourist visiting Slovenia (24). Our patient acquired the disease in 2008 in the Netherlands, where the first domestic case was reported in 1999 (25).
The ubiquitous Q fever, a zoonosis typically transmitted from domesticated mammals to humans by contaminated aerosols, is occasionally found in international travelers (26). Many infected travelers can recall visits to local farms and direct physical contact with domestic animals (27). All of our 11 case-patients had uncomplicated acute Q fever, the typical clinical sign in travelers, and most had a nonfocal febrile illness. However, it should be noted that acute Q fever may be severe and even fatal in travelers (28).
Bartonelloses are rarely reported in travelers but cases of Carrion disease caused by Bartonella bacilliformis have been seen after travel to South America (29,30). In the present series, we identified 7 case-patients likely having B. henselae infection, a ubiquitous condition associated with felines and their fleas and not previously reported in international travelers. Six of our patients had cat-scratch disease, a self-limiting disease characterized by cutaneous papules or pustules at the site at the inoculation site and an often long-lasting painful regional lymphadenitis, and 1 patient had bacillary angiomatosis, a potentially severe condition with vascular proliferation in the skin and internal organs (31).
With an overall incidence rate of 0.6% (and 1.5% of those travelers with fever), our analysis suggests that rickettsial diseases are uncommon in ill returnees. However, underdiagnosis is likely to be widespread, even at specialized institutions such as those associated with the GeoSentinel surveillance network. Few specific clinical signs exist in rickettsial diseases. Many cases have a nonfocal febrile disease of mild-to-moderate severity, accompanied by nonspecific results in routine blood tests (32). The inoculation eschar, a painless black skin lesion surrounded by a red halo that develops at the site of the infective tick or mite bite, is a useful diagnostic clue in SFG rickettsioses and scrub typhus, but may be absent in <40% of such cases (14). The available array of microbiologic diagnostic tests is another predicament in the management of rickettsial diseases. Although sensitive and specific techniques, such as PCR and culture, can be performed at reference centers, most cases worldwide are diagnosed by serologic analysis (1). The principal limitations with serologic analysis include a usually negative result in the acute phase when most patients first seek medical care, poor sensitivity in cases treated early with doxycycline, and an inability to distinguish between the various Rickettsia species caused by cross-reactions (33,34).
Some rickettsial diseases are potentially malignant, but severe complications developed in only a few patients in the present series, and only 1 traveler died. There may be several reasons for this favorable outcome, including a large percentage of benign African tick bite fever cases, and a widespread empiric use of antirickettsial drugs in cases of suspected rickettsial disease. It is noteworthy that rickettsial organisms are inherently resistant to many antimicrobial drugs commonly used as treatment for acute fevers, including the β-lactams, and treatment of choice is tetracyclines, in particular, doxycycline. Fluoroquinolones and newer macrolides may also be useful (1,35).
Our study had limitations similar to those in other GeoSentinel studies, including a possible selection bias towards complicated and unusual cases (3,4). Also, because rickettsial diseases, with the major exceptions of Q fever and bartonelloses, have short incubation periods (typically around 1 week) some cases are likely to manifest during travel and may be seen only by foreign healthcare providers. An analysis solely based on persons reporting posttravel is thus likely to underestimate the true incidence of rickettsial diseases in international travelers. However, because GeoSentinel currently has no site in sub-Saharan Africa, the prime destination for cases of travel-associated rickettsial diseases, we chose to exclude cases seen during travel from the analysis. Lastly, because most ill returnees seen at GeoSentinel sites have traveled to the tropics, we may have underestimated the incidence of rickettsial diseases typically acquired in temperate areas, including Rocky Mountain spotted fever, TIBOLA, and Mediterranean spotted fever.
In summary, the present study demonstrates the wide spectrum of rickettsial diseases that may be encountered in international travelers. Most infections are acquired in sub-Saharan Africa, where African tick bite fever is the predominate disease. The overall outcome is favorable but, because some rickettsial diseases may take a dire course, empirical treatment with an antirickettsial drug should always be considered whenever evaluating a traveler with an otherwise unexplained febrile disease who has recently returned from areas where these diseases are endemic.
Dr Jensenius is a consultant in the Department of Infectious Diseases, Oslo University Hospital, Ullevål, Oslo, Norway. His research interest comprises arthropod-borne diseases in travelers.
Acknowledgments
We thank Elena Axelrod and Adam Plier for technical and organizational support, and David O. Freedman for valuable comments. We are also indebted to all GeoSentinel sites for providing data.
GeoSentinel, the Global Surveillance Network of the International Society of Travel Medicine, is supported by Cooperative Agreement U50/CCU412347 from the Centers for Disease Control and Prevention.
References
- Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–56. DOIPubMedGoogle Scholar
- Jensenius M, Fournier PE, Raoult D. Rickettsioses and the international traveler. Clin Infect Dis. 2004;39:1493–9. DOIPubMedGoogle Scholar
- Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin Infect Dis. 2007;44:1560–8. DOIPubMedGoogle Scholar
- Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–30. DOIPubMedGoogle Scholar
- Tissot-Dupont H, Raoult D. Q fever. Infect Dis Clin North Am. 2008;22:505–14. DOIPubMedGoogle Scholar
- Rutherford JS. Fatal spotted fever rickettsiosis, Kenya. Emerg Infect Dis. 2004;10:910–3.PubMedGoogle Scholar
- Chai JT, Eremeeva ME, Borland CD, Karas JA. Fatal Israeli spotted fever in a UK traveler to south Portugal. J Travel Med. 2008;15:122–3. DOIPubMedGoogle Scholar
- Parola P, Fenollar F, Badiaga S, Brouqui P, Raoult D. First documentation of Rickettsia conorii infection (strain Indian tick typhus) in a traveler. Emerg Infect Dis. 2001;7:909–10. DOIPubMedGoogle Scholar
- Raoult D, Lakos A, Fenollar F, Beytout J, Brouqui P, Fournier PE. Spotless rickettsiosis caused by Rickettsia slovaca and associated with Dermacentor ticks. Clin Infect Dis. 2002;34:1331–6. DOIPubMedGoogle Scholar
- Porta FS, Nieto EA, Creus BF, Espín TM, Casanova FJ, Sala IS, Tick-borne lymphadenopathy: a new infectious disease in children. Pediatr Infect Dis J. 2008;27:618–22. DOIPubMedGoogle Scholar
- Jelinek T, Loscher T. Clinical features and epidemiology of tick typhus in travelers. J Travel Med. 2001;8:57–9.PubMedGoogle Scholar
- Cazorla C, Socolovschi C, Jensenius M, Parola P. Tick-borne diseases: spotted fever group rickettsioses in Africa. Infect Dis Clin North Am. 2008;22:531–44. DOIPubMedGoogle Scholar
- Raoult D, Fournier PE, Fenollar F, Jensenius M, Prioe T, de Pina JJ, Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N Engl J Med. 2001;344:1504–10. DOIPubMedGoogle Scholar
- Jensenius M, Fournier PE, Vene S, Hoel T, Hasle G, Henriksen AZ, African tick bite fever in travelers to rural sub-Equatorial Africa. Clin Infect Dis. 2003;36:1411–7. DOIPubMedGoogle Scholar
- Ding T, Lloyd G, Tolley H, Bradlow A. Tick bite fever and arthritis associated with travel to Africa. Ann Rheum Dis. 2004;63:1703–4. DOIPubMedGoogle Scholar
- Jensenius M, Fournier PE, Fladby T, Hellum KB, Hagen T, Priø T, Sub-acute neuropathy in patients with African tick bite fever. Scand J Infect Dis. 2006;38:114–8. DOIPubMedGoogle Scholar
- Roch N, Epaulard O, Pelloux I, Pavese P, Brion JP, Raoult D, African tick bite fever in elderly patients: 8 cases in French tourists returning from South Africa. Clin Infect Dis. 2008;47:e28–35. DOIPubMedGoogle Scholar
- Azuma M, Nishioka Y, Ogawa M, Takasaki T, Sone S, Uchiyama T. Murine typhus from Vietnam, imported into Japan. Emerg Infect Dis. 2006;12:1466–8.PubMedGoogle Scholar
- Parola P, Vogelaers D, Roure C, Janbon F, Raoult D. Murine typhus in travelers returning from Indonesia. Emerg Infect Dis. 1998;4:677–80. DOIPubMedGoogle Scholar
- Pether JV, Jones W, Lloyd G, Rutter DA, Barry M. Fatal murine typhus from Spain. Lancet. 1994;344:897–8. DOIPubMedGoogle Scholar
- Jensenius M, Montelius R, Berild D, Vene S. Scrub typhus imported to Scandinavia. Scand J Infect Dis. 2006;38:200–2. DOIPubMedGoogle Scholar
- Watt G, Strickman D. Life-threatening scrub typhus in a traveler returning from Thailand. Clin Infect Dis. 1994;18:624–6.PubMedGoogle Scholar
- Bakken JS, Dumler S. Human granulocytic anaplasmosis. [viii. ]. Infect Dis Clin North Am. 2008;22:433–48. DOIPubMedGoogle Scholar
- Laferl H, Hogrefe W, Kock T, Pichler H. A further case of acute human granulocytic ehrlichiosis in Slovenia. Eur J Clin Microbiol Infect Dis. 1999;18:385–6. DOIPubMedGoogle Scholar
- van Dobbenburgh A, van Dam AP, Fikrig E. Human granulocytic ehrlichiosis in western Europe. N Engl J Med. 1999;340:1214–6. DOIPubMedGoogle Scholar
- Ta TH, Jiménez B, Navarro M, Meije Y, González FJ, Lopéz-Veléz R. Q fever in returned febrile travelers. J Travel Med. 2008;15:126–9. DOIPubMedGoogle Scholar
- Potasman I, Rzotkiewicz S, Pick N, Keysary A. Outbreak of Q fever following a safari trip. Clin Infect Dis. 2000;30:214–5. DOIPubMedGoogle Scholar
- Isaksson HJ, Hrafnkelsson J, Hilmarsdóttir I. Acute Q fever: a cause of fatal hepatitis in an Icelandic traveler. Scand J Infect Dis. 2001;33:314–5. DOIPubMedGoogle Scholar
- Matteelli A, Castelli F, Spinetti A, Bonetti F, Graifenberghi S, Carosi G. Short report: verruga peruana in an Italian traveler from Peru. Am J Trop Med Hyg. 1994;50:143–4.PubMedGoogle Scholar
- Lydy SL, Eremeeva ME, Asnis D, Paddock CD, Nicholson WL, Silverman DJ, Isolation and characterization of Bartonella bacilliformis from an expatriate Ecuadorian. J Clin Microbiol. 2008;46:627–37. DOIPubMedGoogle Scholar
- Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics. 2008;121:e1413–25. DOIPubMedGoogle Scholar
- Jensenius M, Fournier PE, Hellum KB, Wesslén L, Caruso G, Priø T, Sequential changes of hematological and biochemical parameters in African tick bite fever. Clin Microbiol Infect. 2003;9:678–83. DOIPubMedGoogle Scholar
- Fournier PE, Jensenius M, Laferl H, Vene S, Raoult D. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin Diagn Lab Immunol. 2002;9:324–8.PubMedGoogle Scholar
- Jensenius M, Fournier PE, Vene S, Ringertz SH, Myrvang B, Raoult D. Comparison of immunofluorescence assay, Western blotting and cross-adsorption for the diagnosis of African tick bite fever. Clin Diagn Lab Immunol. 2004;11:768–83.Medline
- Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48:1921–33. DOIPubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to http://www.medscape.com/cme/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.com. If you are not registered on Medscape.com, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Multicenter GeoSentinel Analysis of Rickettsial Diseases in International Travelers, 1996–2008
CME Questions
1. Which of the following are no longer classified as rickettsial disorders?
A. Ehrlichia and Anaplasma
B. Orientia and Coxiella burnetti
C. Coxiella burnetti and Bartonella
D. Anaplasma and Bartonella
2. A 44-year-old male traveler returning from Tanzania presents 7 days after return with fever and respiratory symptoms. Among rickettsial diseases to be considered, which of the following is most likely to be the cause of his illness?
A. Ehrlichiosis
B. Spotted fever group rickettsiosis
C. Bartonellosis
D. Typhus group rickettsiosis
3. Which of the following is least likely to be positively and independently associated with spotted fever group rickettsiosis in a returning international traveler?
A. Travel for business
B. Visit to southern Africa
C. Male gender
D. Travel from March to May
4. Which of the following is the most commonly used treatment for rickettsial disease among returning international travelers?
A. Tetracycline
B. Minocycline
C. Septra
D. Doxycycline
Activity Evaluation
| 1. The activity supported the learning objectives. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
|
1
|
2
|
3
|
4
|
5
|
| 2. The material was organized clearly for learning to occur. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
|
1
|
2
|
3
|
4
|
5
|
| 3. The content learned from this activity will impact my practice. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
|
1
|
2
|
3
|
4
|
5
|
| 4. The activity was presented objectively and free of commercial bias. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
|
1
|
2
|
3
|
4
|
5
|
In addition to the authors, the following members of the GeoSentinel Surveillance Network contributed data: Kevin C. Kain, University of Toronto, Toronto, Ontario, Canada; Phyllis E. Kozarsky and Carlos Franco-Paredes, Emory University, Atlanta, Georgia, USA; Louis Loutan and François Chappuis, University of Geneva, Geneva, Switzerland; Joseph Torresi and Graham Brown, Royal Melbourne Hospital, Melbourne, Victoria, Australia; DeVon C. Hale and Stefanie S. Gelman, University of Utah, Salt Lake City, Utah, USA; Alice Pérignon, Hôpital Pitié-Salpêtrière, Paris, France; Gerd-Dieter Burchard, Bernhard-Nocht-Institute for Tropical Medicine, Hamburg, Germany; Mary E. Wilson, Harvard University, Cambridge, Massachusetts, USA; Fabrice Simon and Jean Delmont, Hôpital Nord, Marseille, France; William M. Stauffer and Patricia F. Walker, University of Minnesota, Minneapolis, Minnesota, USA; Poh Lian Lim and Annelies Wilder-Smith, Tan Tock Seng Hospital, Singapore; Jose Antonio Perez Molina, Hospital Ramon y Cajal, Madrid, Spain; Bradley A. Connor, Cornell University, Ithaca, New York, USA; Carmelo Licitra and Antonio Crespo, Orlando Regional Health Center, Orlando, Florida, USA; David O. Freedman, University of Alabama at Birmingham, Birmingham, Alabama, USA; Effrossyni Gkrania-Klotsas, Addenbrooke's Hospital, Cambridge, UK; Giampiero Carosi and Francesco Castelli, University of Brescia, Brescia, Italy; Marc Shaw, Worldwise Travelers Health and Vaccination Centre, Auckland, New Zealand; Prativa Pandey, CIWEC Clinic Travel Medicine Center, Kathmandu, Nepal; R. Bradley Sack and Robin McKenzie, Johns Hopkins University, Baltimore, Maryland, USA (Dec 1997–Aug 2007); Elizabeth D. Barnett, Boston University, Boston, Massachusetts, USA; Christina M. Coyle and Murray Wittner, Albert Einstein School of Medicine, Bronx, New York, USA; Stefan Hagmann and Andy Miller, Bronx-Lebanon Hospital Center, Bronx; Michael W. Lynch, Fresno International Travel Medical Center, Fresno, California, USA; Vanessa Field, InterHealth, London, UK; Michael D. Libman and J. Dick Maclean, McGill University, Montreal, Quebec, Canada; Alejandra Gurtman, Mount Sinai Medical Center, New York, New York, USA (Oct 2002–Aug 2005); Shuzo Kanagawa and Yasuyuki Kato, International Medical Center of Japan, Tokyo, Japan; and Patricia Schlagenhauf, Rainer Weber, and Robert Steffen, University of Zürich, Zürich, Switzerland.
Related Links
Table of Contents – Volume 15, Number 11—November 2009
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Mogens Jensenius, Department of Infectious Diseases, Oslo University Hospital, Ullevål, NO-0407 Oslo, Norway
Top