Volume 15, Number 7—July 2009
Dispatch
WU Polyomavirus in Patients Infected with HIV or Hepatitis C Virus, Connecticut, USA, 2007
Figure
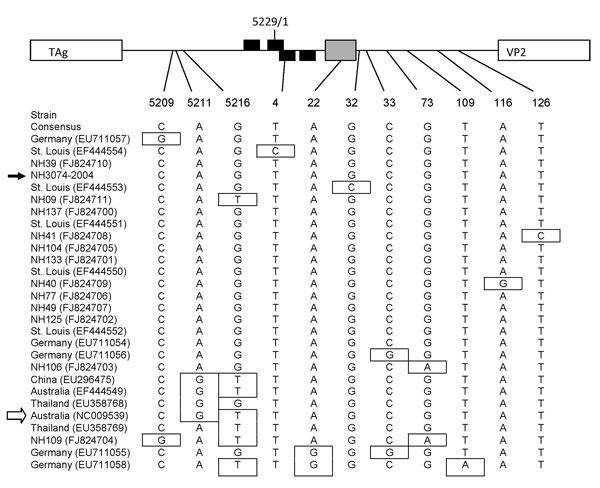
Figure. Nucleotide polymorphisms in the noncoding region of WU polyomavirus (WUPyV), Connecticut, USA, 2007. Sequences spanning nt 5197 to 159 of the circular viral genome of New Haven human serum isolates and all available sequences from GenBank (all from respiratory specimens) were subjected to phylogenetic analysis. A map of the noncoding region within the viral genome is indicated at the top of the figure (not to scale). The arbitrary last (5229) and first (1) nucleotide of the circular viral genome are indicated. The putative large T antigen (TAg) binding sites for each strand of the double-strand genome are indicated by black boxes, and the A/T-rich region is indicated by a gray box. Position of nucleotide polymorphism is indicated below the map. Strains (location and GenBank accession nos.) are listed in order according to phylogenic analysis. The consensus sequence is listed first, and polymorphisms are indicated by boxes. The strain indicated by the black arrow is a WUPyV New Haven respiratory isolate (6), and the strain indicated by the white arrow is the original WUPyV isolate (2). VP2, virus capsid protein 2.
References
- Allander T, Andreasson K, Gupta S, Bjerkner A, Gordana B, Perrson MA, Identification of a third human polyomavirus. J Virol. 2007;81:4130–6. DOIPubMedGoogle Scholar
- Gaynor AM, Nissen MD, Whiley DM, MacKay IM, Lambert SB, Wu G, Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. DOIPubMedGoogle Scholar
- Norja P, Ubillos I, Templeton K, Simmonds P. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J Clin Virol. 2007;40:307–11. DOIPubMedGoogle Scholar
- Le BM, Demertzis LM, Wu G, Tibbets RJ, Buller R, Arens MQ, Clinical and epidemiologic characterization of WU polyomavirus infection, St. Louis, Missouri. Emerg Infect Dis. 2007;13:1936–8.PubMedGoogle Scholar
- Bialasiewicz S, Whiley DM, Lambert SB, Jacob K, Bletchly C, Wang D, A newly reported human polyomavirus, KI virus, is present in the respiratory tract of Australian children. J Clin Virol. 2007;40:15–8. DOIPubMedGoogle Scholar
- Wattier RL, Vazquez MV, Weibel C, Shapiro ED, Ferguson D, Landry ML, Role of human polyomaviruses in respiratory tract disease in young children. Emerg Infect Dis. 2008;14:1766–8. DOIPubMedGoogle Scholar
- Khalili K, Gordon J, White MK. The polyomavirus, JCV and its involvement in human disease. Adv Exp Med Biol. 2006;577:274–87. DOIPubMedGoogle Scholar
- Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25–30. DOIPubMedGoogle Scholar
- Andreoletti L, Lescieux A, Lambert B, Si-Mohamed A, Matta M, Wattre P, Semiquantitative detection of JCV-DNA in peripheral blood leukocytes from HIV-1-infected patients with or without progressive multifocal leukoencephalopathy. J Med Virol. 2002;66:1–7. DOIPubMedGoogle Scholar
- Hymes LC, Warshaw BL. Polyomavirus (BK) in pediatric renal transplants: evaluation of viremic patients with and without BK associated nephritis. Pediatr Transplant. 2006;10:920–2. DOIPubMedGoogle Scholar
- Stolt A, Sasnauskas K, Koskela P, Lehtinen M, Dillner J. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84:1499–504. DOIPubMedGoogle Scholar
- Costa C, Bergallo M, Astegiano S, Terlizzi ME, Sidoti F, Segoloni GP, Monitoring of BK virus replication in the first year following renal transplantation. Nephrol Dial Transplant. 2008;23:3333–6. DOIPubMedGoogle Scholar
- Sharp CP, Norja P, Anthony J, Bell JE, Simmonds P. Reactivation and mutation of newly discovered WU, KI, and Merkel cell carcinoma polyomaviruses in immunosuppressed individuals. J Infect Dis. 2009;199:398–404. DOIPubMedGoogle Scholar
- zur Hausen H. Novel human polyomaviruses–re-emergence of a well known virus family as possible human carcinogens. Int J Cancer. 2008;123:247–50. DOIPubMedGoogle Scholar
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100. DOIPubMedGoogle Scholar