Volume 15, Number 9—September 2009
Letter
Cross-reactive Antibodies against Avian Influenza Virus A (H5N1)
Cite This Article
Citation for Media
To the Editor: Intravenous immunoglobulin (IVIg) is used to treat bacterial and viral infections in patients with primary immunodeficiency disease and those with autoimmune and inflammatory disorders (1). IVIg contains pooled IgG from >1,000 blood donors and antibodies against various common human pathogens, including influenza virus A.
We tested the efficacy of commercial preparations of IVIg (50 mg/mL of highly purified immunoglobulin) against homosubtypic influenza viruses A (H1N1 and H3N2) and their cross-reactivity against avian influenza virus A (H5N1). IVIg preparations (Octagam; Octapharma, Vienna, Austria and Flebogamma; Instituto Grifols, Barcelona, Spain) had hemagglutination inhibition (HI) titers against subtypes H1N1 and H3N2 ranging from 20 to 40. Human Immunoglobulin, pH 4.0, (Harbin Sequel Bio-Engineering Pharmaceutical, Harbin, People’s Republic of China) had lower HI titers against homosubtypic avian influenza viruses (10 for subtype H3N2 and <10 for subtype H1N1). As expected, we did not detect antibodies against hemagglutinin (HA) of subtype H5N1 (A/open-billed/stork/Nahkonsawan/BBD0104F/2004) in any of the IVIg preparations (HI titer <10).
Human influenza subtype H1N1 shares the same neuraminidase (NA) subtype (human N1) as subtype H5N1 (avian N1). We therefore tested whether IVIg preparations would react and inhibit NA activity of human and avian influenza viruses by using a neuraminidase inhibition (NI) assay (2). NI titer was defined as the reciprocal of the highest dilution that gave 50% reduction compared with that of the virus control.
All 3 IVIg preparations inhibited NA activity of human N1 (NI titer against subtype H1N1 range 258–986) and human N2 (NI titer against subtype H3N2 range 1,309–3,274). Enzyme activity of avian N1 (7:1 reassortant; PR8 + NA [A/Vietnam/DT-0361/2005 H5N1]) was inhibited by all IVIg preparations (NI titer range 143–231). These findings support the recent observation of neutralizing antibodies against human N1 in human serum, which could inhibit enzyme activity of avian N1 from subtype H5N1 (3,4). We also tested IVIg preparations against reverse genetics subtype H5N3 virus in which the N3 NA was derived from H2N3 virus (6:1:1 reassortant; 6 internal genes from PR8 + HA (A/Vietnam/DT-0361/05 H5N1) + NA (A/duck/Germany 1207 H2N3) and observed no effect (NI titer <10). The N3 subtype belongs to avian influenza NA. Thus, antibodies against NA in IVIg appear to be specific for those circulating human influenza viruses (human N1 and human N2).
Unlike HA and NA, virus matrix 2 ectodomain (M2e) is highly conserved. Its presence on the surface of the viral particle makes it a potential target of antibody response similar to that for HA and NA (5,6). We assessed reactivity of IVIg preparations against a consensus M2e peptide derived from human influenza viruses of H1, H2, and H3 subtypes (MSLLTEVETPIRNEWGCRCNDSSD) and those derived from A/Hong Kong/156/97 H5N1 (MSLLTEVETLTRNGWGCRCSDSSD and A/Thailand/ SP-83/2004 H5N1 (MSLLTEVETPTRNEWECRCSDSSD) by using ELISA (7). Antibody titer was defined as the reciprocal of the highest dilution that had an optical density of 0.5 at 414 nm in our assay.
Results showed considerable variation among IVIg preparations, caused by M2e peptides derived from different influenza viruses (titer range 88–23,614). Among the 3 preparations, Human Immunoglobulin, pH 4.0, IVIg showed the highest titers against all M2e peptides (consensus, 9,639; H5N1 Hong Kong, 3,519; and H5N1 Thailand, 23,614). Variation of antibody titers against M2e in IVIGs may be geographically dependent. Unlike Octagam and Flebogamma, Human Immunoglobulin, pH 4.0, IVIg was likely derived from blood donors in China. Octagam and Immunoglobulin, pH 4.0, IVIg were more reactive with M2e of avian influenza virus (H5N1) (A/Thailand/SP-83/2004) than with other M2e peptides.
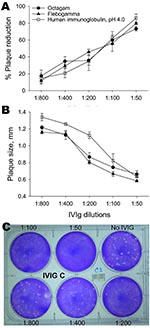
We measured the ability of IVIg preparations to inhibit influenza subtype H5N1 replication by using a plaque-reduction assay. Subtype H5N1 (A/open-billed stork/ Nakhonsawan/BBD0104F/2004) was maintained as described (8). MDCK cells were infected with virus and agar containing various concentrations of IVIg was layered on top of these cells and incubated for 2 days. Results are shown in the Figure. IVIG inhibited plaque formation in a dose-dependent manner. Although plaques of heterogeneous size were observed in infected plates without IVIg, larger plaques were preferentially neutralized with increasing concentrations of IVIg in the agar (Figure).
Premixing excess M2e peptide with IVIg to absorb M2e-specific antibodies had no effect on plaque formation, indicating that antibodies against M2e in IVIg preparations were not responsible for neutralization of influenza subtype H5N1. Antibodies against M2e may have a role in protection against subtype H5N1 by another mechanism.
Our data suggest that the neutralizing activity against influenza subtype H5N1 in all 3 IVIg preparations was likely contributed by cross-reactive antibodies against avian N1. IVIg has been reported to have antiinflammatory activity (9,10). The immune suppressive effect of IVIg may benefit patients by reducing the cytokine storm. These data suggest use of IVIg, especially preparations containing high neutralizing activity against subtype H5N1, as adjunctive treatment for infection with highly pathogenic avian influenza virus (H5N1).
Acknowledgment
This study was supported by grant Y1-AI-5026-01 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, International Research in Infectious Disease.
References
- McClelland DB, Yap PL. Clinical use of immunoglobulins. Clin Haematol. 1984;13:39–74.PubMedGoogle Scholar
- Lambre CR, Terzidis H, Greffard A, Webster RG. Measurement of anti-influenza neuraminidase antibody using a peroxidase-linked lectin and microtitre plates coated with natural substrates. J Immunol Methods. 1990;135:49–57. DOIPubMedGoogle Scholar
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4:e59. DOIPubMedGoogle Scholar
- Lynch GW, Selleck PW, Axell A-M, Downton T, Kapitza NM, Boehm I, Cross-reactive anti-avian H5N1 influenza neutralizing antibodies in a normal ‘exposure-naive’ Australian blood donor population. The Open Immunology Journal. 2008;1:13–9. DOIGoogle Scholar
- Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–63. DOIPubMedGoogle Scholar
- Zharikova D, Mozdzanowska K, Feng J, Zhang M, Gerhard W. Influenza type A virus escape mutants emerge in vivo in the presence of antibodies to the ectodomain of matrix protein 2. J Virol. 2005;79:6644–54. DOIPubMedGoogle Scholar
- Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z, Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13:426–35. DOIPubMedGoogle Scholar
- Thitithanyanont A, Engering A, Ekchariyawat P, Wiboon-ut S, Limsalakpetch A, Yongvanitchit K, High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J Immunol. 2007;179:5220–7.PubMedGoogle Scholar
- Ephrem A, Misra N, Hassan G, Dasgupta S, Delignat S, Van Huyen JP, Immunomodulation of autoimmune and inflammatory diseases with intravenous immunoglobulin. Clin Exp Med. 2005;5:135–40. DOIPubMedGoogle Scholar
- Ephrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111:715–22. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 15, Number 9—September 2009
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sathit Pichyangkul, Department of Immunology and Medicine, US Army Medical Component, Armed Forces Research Institute of Medical Sciences, 315/6 Rajvithi Rd, Bangkok, 10400, Thailand;
Top