Volume 16, Number 11—November 2010
Research
Effect of Vaccination on Bordetella pertussis Strains, China
Abstract
Whole-cell pertussis vaccine was introduced in China in the early 1960s. We used standard typing methods to compare 96 Bordetella pertussis isolates collected before and after introduction of vaccination, during 1953–2005. The following vaccine-type alleles of the pertussis toxin (ptx) gene were characteristic for all prevaccination strains: ptxA2, ptxA3, and ptxA4. The shift to ptxA1 occurred since 1963. All isolates collected since 1983 contained ptxA1. Pertactin (prn) allele 1, prn1, was predominant, although prn2 and prn3 have been detected since 2000. Serotypes fimbriae (Fim) 2 and Fim2,3 were found in all isolates collected before 1986. During 1997–2005, Fim3 became prevalent. Although changes in electrophoresis profiles over time were observed, the predominant profiles during 1997–2005 resembled those during the prevaccine era and those found in Europe before the 1990s. B. pertussis strains in China may differ from those in countries that have a long history of high vaccine coverage.
Whooping cough (pertussis) is an acute respiratory infectious disease caused by the bacterium Bordetella pertussis. After the whole-cell pertussis (Pw) vaccines were introduced in many countries during 1940–1960, illness and death rates from pertussis have decreased dramatically (1,2). However, pertussis remains a leading cause of vaccine-preventable deaths worldwide (1). A resurgence of pertussis has been observed in developed countries despite high vaccination coverage (3–9).
In the People’s Republic of China, vaccination against pertussis was started in the early 1960s, when 3 doses of Pw vaccine combined with diphtheria and tetanus toxoids were given at 3, 4, and 5 months of age. In 1982, a booster dose at 18 months of age was added (10,11). Pw vaccines are free of charge in China. Since 1995, acellular pertussis (Pa) vaccines containing purified pertussis toxin (Ptx) and filamentous hemagglutinin have been also introduced. However, because Pa vaccines are offered at the patient’s expense, use of these vaccines has been minimal, especially in some resource-limited areas. Although since 2007 Pa vaccines have been included in the national expanded program on immunization, Pa and Pw vaccines are still used in most provinces because of limited availability and cost of Pa vaccines. Although the reported vaccination coverage for the primary 3 doses increased with time, before the 1980s it was low. From 1983 through 2008, coverage ranged from 58% to 99% (median 93%) (Figure 1) (12).
In China, pertussis is a reportable infectious disease, and the number of reported cases has been decreasing (Figure 1). Since the 1990s, incidence has been <1 case/100,000 population (12). In China, pertussis is clinically diagnosed by physicians; laboratory methods such as culture, PCR, and serologic analysis are not used for diagnosis of pertussis. Therefore, the reported low incidence may be related to the diagnostic criteria used, suggesting substantial underreporting. Pertussis remains endemic to China (10–12), and a local outbreak was reported in April 1997.
In many countries, divergence in major antigens Ptx, pertactin (Prn), fimbriae (Fim) 2, Fim3, and tracheal colonization factor (TcfA) between the vaccine strains and circulating isolates has been reported (3,4,7,9,13–16). Furthermore, marked changes in B. pertussis strains have been found in these countries after introduction of vaccination. To learn more about the B. pertussis strains circulating in China, we used standardized typing methods to analyze and compare B. pertussis isolates collected before and after the introduction of vaccination (17).
Bacterial Strains and Culture
We tested 3 vaccine strains and 96 clinical isolates: 25 isolates from 1953–1958, 52 from 1963–1985, and 19 from 1997–2005. These 3 periods were chosen according to when Pw vaccines were introduced (1960) and when Pa vaccines were added to the vaccination program (1995). Information on vaccine strains and clinical isolates is shown in Appendix Figure 1 and in the Technical Appendix. Clinical information for patients from whom B. pertussis was isolated was not available. Vaccine strains P3s10 and CS were isolated in Beijing in 1951 (Appendix Figure 1). Vaccine strain 18530 originated in the United States. Strains P3s10 and 18530 have been used to produce Pw vaccines since the early 1960s, and strain CS has been used to produce Pa vaccines since 1995. All B. pertussis isolates were confirmed by slide agglutination with specific antiserum to B. pertussis and B. parapertussis (Murex Diagnostics, Dartford, UK) and by PCR according to insertion sequence IS481 (18). B. pertussis strains were grown on Bordet-Gengou agar supplemented with 15% defibrinated sheep blood, incubated at 37°C for 4–5 days.
Serotyping
Serotyping was performed by a microtiter plate–based monoclonal agglutination assay as described (17). Monoclonal antibodies against Fim2 (NIBSC 04/154) or Fim3 (NIBSC 04/156) were used.
DNA Sequencing
PCR-based sequencing of 5 genes (prn, ptxA, tcfA, fim2, and fim3) was performed as described (17,19,20) with minor modifications. Nucleotide sequences were determined for the complete opening read frames of ptxA, fim2, fim3, and tcfA, and for part of prn. The same primers were used for amplification and sequencing (Table). The sequencing assays were performed with an ABI Prism 3100 DNA sequencing system (Applied Biosystems, Foster City, CA, USA), and data analysis was conducted with DNASTAR (Madison, WI, USA) software.
Pulsed-Field Gel Electrophoresis
Pulsed-field gel electrophoresis (PFGE) was performed as recommended (17) with minor modifications. Briefly, after the DNA plugs were treated with 50 U of XbaI (New England Biolabs, Ipswich, MA, USA), the cleaved DNA fragments were separated by electrophoresis on a 1% agarose gel by using a Chef Mapper (Bio-Rad, Hercules, CA, USA) with pulse times of 2.16–44.69 s for 24 h. The band patterns were analyzed with BioNumerics program version 4.0 (Applied-Maths, Kortrijk, Belgium). The clustering method used was the unweighted pair group method with arithmetic mean. The Pertussis Reference Laboratory of the National Institute for Health and Welfare, Turku, Finland, used PFGE to retest 53 clinical isolates, 3 vaccine strains, and international reference strain 18323 (7).
The selection criteria for the 53 isolates included at least 1 strain for 1 unique profile. If 2 strains had identical profiles, both were retested. If multiple strains represented the same profile, the strains isolated in different years or different regions were included. For PFGE, 8 international reference strains were included. The nomenclature was based on the profiles already defined for Finland (BpFINR) and Sweden (BpSR) (7,21). Profiles assigned as BpCHR have been found only among the analyzed isolates from China. A 2-sided Fisher exact test was used to compare frequencies of strain serotypes and genotypes from the 3 periods.
Fimbrial Serotypes
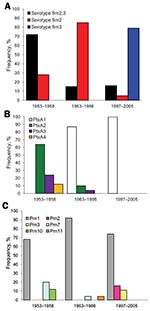
Vaccine strains P3s10 and CS were serotype Fim2,3, and vaccine strain 18530 was serotype Fim3. Among the clinical isolates, all 3 serotypes were found (Figure 2, panel A). Significantly more isolates collected during 1963–1986 were serotype Fim2 than were isolates collected during 1953–1958 (p<0.001). Of the 19 isolates collected during 1997–2005, we found that 15 (79%) were Fim3, 3 (16%) were Fim2,3, and 1 (5%) was Fim2. Significantly more isolates collected during 1997–2005 were serotype Fim3 than were isolates collected during 1953–1958 and 1963–1986 (p<0.001 for each).
Alleles of ptxA, prn, tcfA, fim2, and fim3
Vaccine strains P3s10 and CS contained ptxA2/prn1/tcfA2/fim2–1/fim3–1, whereas vaccine strain 18530 contained ptxA3/prn1/tcfA2/fim2–2/fim3–1. Among the clinical isolates, all 4 ptxA alleles (ptxA1, ptxA2, ptxA3, and ptxA4) were found (Figure 2, panel B). However, the frequency of each allele changed over time. In the prevaccine era, 64% (n = 16), 24% (n = 6), and 12% (n = 3) of isolates contained ptxA2, ptxA3, and ptxA4, respectively. The allele ptxA1 appeared in 1963 and has become predominant since then. After the 1980s, all isolates contained ptxA1.
Altogether 6 prn alleles (prn1, prn2, prn3, prn7, prn10, and prn11) were detected. The amino acid sequences in variable regions of the 6 alleles are shown in Appendix Figure 2. Allele prn1 remained predominant at 81% (78/96) during the study period (Figure 2, panel C). No significant difference was found in the frequency of finding prn2 and prn3 in isolates collected during 1997–2005 than in those collected during 1953–1958 (p = 0.749) and 1963–1986 (p = 0.0513). All 7 isolates with prn7 contained ptxA3, whereas all 3 isolates with prn10 contained ptxA4. Two isolates with prn11 contained ptxA2. All 5 isolates with prn2 or prn3 contained ptxA1.
Four tcfA alleles (tcfA1, tcfA2, tcfA5, and tcfA9) were identified. Allele tcfA2 was most common, representing 94% (n = 90) of the isolates (Technical Appendix). Also detected were 2 fim2 (fim2–1 and fim2–2) and 3 fim3 (fim3–1, fim3–2, and fim3–4) alleles. Of the 96 isolates, 90 (94%) contained fim2–1. All 6 isolates with fim2–2 were recovered during 1953–1958. Five of the 6 isolates with fim2–2 contained prn7. For the fim3 alleles, 96% of isolates were fim3–1. All 3 isolates with fim3–2 and 1 isolate with fim3–4 were recovered in 1997–2005. Identical amino acid sequences were found for fim3–1 and fim3–4, but a silent mutation at nt 87 was found for fim3–4. All 3 isolates harboring fim3–2 contained prn2.
PFGE Profiles
Vaccine strains P3s10 and CS had an identical profile, BpCHR6, whereas vaccine strain 18530 represented BpFINR13 (Figure 3). The 96 isolates produced 27 distinct profiles, 4 of which (BpFINR9, BpSR127, BpSR23, and BpSR11) have been reported in Europe. The PFGE profiles obtained from vaccine strain 18530 (BpFINR13) and international reference strain 18323 were not detected among the isolates from China. The 6 common profiles represented 70% of isolates (41 isolates with BpCHR15, 7 with BpCHR6, 6 with BpCHR2, 5 with BpSR127, 5 with BpSR23, and 3 with BpCHR20). The PFGE profiles changed over time. Dendrogram analysis of the 27 profiles indicated that they belonged to 8 clusters. Among these clusters, 5 (clusters I–IV and VII) have been reported in Europe (5,22,23).
Previous studies have shown that cluster I includes international reference strain 18323 and 1 clinical isolate (21,22). Clusters II and III include the vaccine strains and most strains that were circulating before the 1990s. Cluster IV contains most strains currently circulating in Europe. Cluster VII, a new group, consists of some isolates collected from Finland in 2004 (23). Of the 3 clusters identified in our study, 1 consisted of 4 profiles (BpCHR1–3 and BpFIN13). Profile BpFINR13 was found only in pertussis vaccine strain 18530 (7). The strain was obtained from the United States and used as a vaccine strain in Finland and China. The second cluster contained profiles BpCHR14 and BpCHR19, and the third contained profiles BpCHR20 and BpCHR22.
In this study, 56 isolates tested belonged to cluster III (Figure 3). Cluster III contained 6 profiles (BpCHR8, BpCHR15, BpCHR17, BpFINR9, BpSR23, and BpSR127) with a minimum of 79% overall relatedness. All isolates belonging to the cluster were collected during 1963–1986 and 1997–2005. Of the 96 clinical isolates, only 2 isolates that belonged to cluster IV (IV-β) were identified; these 2 isolates had PFGE profile BpSR11. Strains with BpSR11 were first detected in France in 1996 (22) and have been prevalent in Europe since then (13). The 2 isolates with BpSR11 were recovered in 2000 and 2001 and contained prn2.
Few B. pertussis isolates from China contained nonvaccine type alleles prn2 or prn3; those that did were found later. In many countries, the prn1 allele is found in most vaccine strains and predominated before introduction of vaccination. However, the vaccine type strains were gradually replaced by nonvaccine type strains, mainly with allele prn2, after the introduction of vaccination. The most prevalent type in modern isolates is prn2 (7,14,20,24). In Taiwan, Pw vaccines have been offered since 1954 (25). When 168 clinical isolates collected in Taiwan during 1993–2004 were analyzed, prn2 strains accounted for 39% in 1993–1996 and increased to 90% in 1998–2004. In contrast to findings for many countries with long histories of high vaccination coverage, prn2 was first found in China in 2000. The exact reasons for the low frequency of strains with prn2 and their relatively late emergence in China are not known. One explanation might be the low vaccination coverage in China before the 1980s and differences in vaccine coverage between urban and rural areas. Another reason might be the closed borders.
In Japan, divergence of Prn and Ptx between vaccine strains and circulating isolates (26–28) has been reported. Pw vaccines were introduced in Japan in 1958; the vaccine strain used was prn1/ptxA2. In 1971, reported vaccination coverage was 50% (8). In 1976, vaccination coverage dropped to only 9%, and pertussis returned. In 1981, a Pa vaccine containing Ptx and filamentous hemagglutinin was introduced in Japan (27). The strain used for production of the Pa vaccines was Tohama I (prn1/ptxA2), isolated in the 1950s. When 107 isolates collected from 1988 through 2001 were studied, the nonvaccine type prn2/ptxA1 appeared in 1994 and was found in 27%–42% of isolates from 1994 through 2001 (26). A recent study reported similar frequency (33%) of the nonvaccine type prn2/ptxA1 in Japan when 60 isolates collected during 1991–2007 were analyzed (28).
TcfA has been shown to be crucial for B. pertussis colonization (29). A total of 9 tcfA alleles have been reported (30,31), and the most common allele is tcfA2 (20,24). Our finding that 94% of isolates studied contained tcfA2 agreed with findings from earlier studies (20,24). Allele tcfA1 has been described only for reference strain 18323. In our study, 3 clinical isolates recovered during 1953–1958 were found to contain tcfA1. All 3 isolates were recovered from the same geographic area. Allele tcfA1 contains a 75-bp segment not found in other tcfA variants. The strain with tcfA1 was postulated to be the progenitor of the strains with tcfA2, tcfA3, or tcfA5 (20).
Several studies have demonstrated that serotype Fim3 is predominant in vaccinated populations, whereas serotypes Fim2 or Fim2,3 are predominant in unvaccinated populations (14,32,33). In Sweden, before 1979 when Pw vaccines were first used, 70% of circulating strains were serotype Fim3 (32). During 1979–1995, when pertussis vaccination was stopped, Fim2 started to increase and reached 64% in the early 1990s. In 1996, when general vaccination with Pa vaccines was reintroduced, prevalence of Fim2 declined and Fim3 strains emerged rapidly. In 2002 and 2003, Fim3 was found in 96% of fully vaccinated persons. In China, before introduction of vaccination, the prevalent serotypes were Fim2,3 and Fim2. After vaccination, the frequency of serotype Fim2,3 decreased and Fim2 became predominant. The possible explanation for the predominance of Fim2 strains after vaccination is that the 2 vaccine strains used in China express Fim2,3 and Fim3. The shift from serotype Fim2 to Fim3 was observed in 1998 and coincided with the introduction of Pa vaccines in this country. Pa vaccines without fimbrial antigens may have some effect on fimbrial serotypes of circulating isolates, as was observed in Sweden (32); however, the exact reason remains to be shown.
In our study, most strains from China had different PFGE profiles than did those from Europe. However, many PFGE profiles detected among the strains from China fell into the same clusters as those reported in Europe (5,22). For example, the most common profile, BpCHR15, fell into the same cluster (cluster III) as BpSR23 and BpSR127 (14). Cluster III includes most strains circulating in Europe before the 1990s (5,32). In China, strains with BpCHR15 had been prevalent during 1965–1983. Although the strains with BpCHR15 were recovered from several different regions and over 20 years, the possibility that some strains were isolated during outbreaks cannot be excluded.
The major PFGE cluster circulating in Europe since the 1990s is cluster IV (5,32). Cluster IV can be divided into 3 subgroups (α, β, and γ), the frequency of which differs among countries. However, since the late 1990s in many countries in Europe, subgroup IV-β became more prevalent than the other 2 subgroups (13). In our study, only 2 isolates with BpSR11 belonged to group IV-β, whereas no isolates fell into subgroups IV-α or IV-γ. This PFGE result correlates with genotyping results.
When we examined the association of 6 common PFGE profiles with different allele combinations, we found that of the 51 isolates with BpCHR15, BpSR23, or BpSR127, 94% contained ptxA1/prn1/tcfA2/fim2–1/fim3–1. Of the 10 isolates with BpCHR6 or BpCHR20, 100% contained ptxA2/prn1/tcfA2/fim2–1/fim3–1. Of the 6 isolates with BpCHR2, all contained ptxA3/prn7/tcfA2/fim2–2/fim3–1.
The emergence of B. pertussis strains carrying a novel allele (ptxP3) for the Ptx promoter has been recently observed in countries with long histories of high vaccination coverage, such as the Netherlands (34). Furthermore, all strains from the Netherlands with BpSR11 were found to carry the ptxP3 allele. In our study, only 2 isolates from China were found to have BpSR11, suggesting that ptxP3 strains are not common in China.
The limitations of this study include the small number of B. pertussis isolates collected during the study period and recent isolates collected mainly from Beijing and its surrounding area. Therefore, our data should be interpreted with caution, and more epidemiologic studies with a larger number of isolates should be conducted in this country.
In conclusion, B. pertussis strains in China may differ from those in countries with long histories of high vaccination coverage. Continuous monitoring of B. pertussis strains is needed.
Liu Zhang is a master of science student at the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China. Her research interests are the epidemiology and molecular biology of B. pertussis.
Acknowledgments
We thank Yue Ma and Jingyun Li for excellent technical assistance and Dorothy Xing for providing monoclonal antibodies against Fim2 (NIBSC 04/154) and Fim3 (NIBSC 04/156).
This work was supported by the National Science & Technology Pillar Program from the Ministry of Science and Technology, China (no. 2008BAI54B03) and in part by the Academy of Finland.
References
- Kerr JR, Matthews RC. Bordetella pertussis infection: pathogenesis, diagnosis, management, and the role of protective immunity. Eur J Clin Microbiol Infect Dis. 2000;19:77–88. DOIPubMedGoogle Scholar
- Galanis E, King AS, Varughese P, Halperin SA; IMPACT investigators. Changing epidemiology and emerging risk groups for pertussis. CMAJ. 2006;174:451–2. DOIPubMedGoogle Scholar
- Mooi FR, He Q, van Oirschot H, Mertsola J. Variation in the Bordetella pertussis virulence factors pertussis toxin and pertactin in vaccine strains and clinical isolates in Finland. Infect Immun. 1999;67:3133–4.PubMedGoogle Scholar
- De Melker HE, Schellekens JF, Neppelenbroek SE, Mooi FR, Rümke HC, Conyn-van Spaendonck MA. Reemergence of pertussis in the highly vaccinated population of the Netherlands: observations on surveillance data. Emerg Infect Dis. 2000;6:348–57. DOIPubMedGoogle Scholar
- Caro V, Njamkepo E, Van Amersfoorth SC, Mooi FR, Advani A, Hallander HO, Pulsed-field gel electrophoresis analysis of Bordetella pertussis populations in various European countries with different vaccine policies. Microbes Infect. 2005;7:976–82. DOIPubMedGoogle Scholar
- Güriş D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clin Infect Dis. 1999;28:1230–7. DOIPubMedGoogle Scholar
- Elomaa A, Advani A, Donnelly D, Antila M, Mertsola J, Hallander H, Strain variation among Bordetella pertussis isolates in Finland, where the whole-cell pertussis vaccine has been used for 50 years. J Clin Microbiol. 2005;43:3681–7. DOIPubMedGoogle Scholar
- Godfroid F, Denoël P, Poolman J. Are vaccination programs and isolate polymorphism linked to pertussis re-emergence? Expert Rev Vaccines. 2005;4:757–78. DOIPubMedGoogle Scholar
- Elomaa A, Advani A, Donnelly D, Antila M, Mertsola J, He Q, Population dynamics of Bordetella pertussis in Finland and Sweden, neighbouring countries with different vaccination histories. Vaccine. 2007;25:918–26. DOIPubMedGoogle Scholar
- Zhang XL, Yang ZW, Zhou J, Yu JJ, Wang KA. An analysis of current epidemiological characteristics of Bordetella pertussis in China [in Chinese]. Chin J Vac Immun. 2000;6:93–5.
- Wang J, Yang Y, Li J, Mertsola J, Arvilommi H, Shen X, Infantile pertussis rediscovered in China. Emerg Infect Dis. 2002;8:859–61.PubMedGoogle Scholar
- World Health Organization. Immunization, vaccination and biologicals. Vaccine preventable diseases monitoring system: 2009 global summary [cited 2010 Feb 26]. http://www.who.int/immunization_monitoring/en/globalsummary/countryprofileselect.cfm
- Hallander H, Advani A, Riffelmann M, von König CH, Caro V, Guiso N, Bordetella pertussis strains circulating in Europe in 1999 to 2004 as determined by pulsed-field gel electrophoresis. J Clin Microbiol. 2007;45:3257–62. DOIPubMedGoogle Scholar
- Kallonen T, He Q. Bordetella pertussis strain variation and evolution postvaccination. Expert Rev Vaccines. 2009;8:863–75. DOIPubMedGoogle Scholar
- Mooi FR, van Oirschot H, Heuvelman K, van der Heide HG, Gaastra W, Willems RJ. Polymorphism in the Bordetella pertussis virulence factors P.69: pertactin and pertussis toxin in the Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect Immun. 1998;66:670–5.PubMedGoogle Scholar
- Fry NK, Neal S, Harrison TG, Miller E, Matthews R, George RC. Genotypic variation in the Bordetella pertussis virulence factors pertactin and pertussis toxin in historical and recent clinical isolates in the United Kingdom. Infect Immun. 2001;69:5520–8. DOIPubMedGoogle Scholar
- Mooi FR, Hallander H, Wirsing von König CH, Hoet B, Guiso N. Epidemiological typing of Bordetella pertussis isolates: recommendations for a standard methodology. Eur J Clin Microbiol Infect Dis. 2000;19:174–81. DOIPubMedGoogle Scholar
- Xu YH, Xu YQ, Zhang SM, Wang LC, Hou QM, Lei DL. Development of fluorescence quantitative PCR for detection of Bordetella pertussis and its application [in Chinese]. J Lab Med. 2008;6:690–4.
- Tsang RS, Lau AK, Sill ML, Halperin SA, Van Caeseele P, Jamieson F, Polymorphisms of the fimbria fim3 gene of Bordetella pertussis strains isolated in Canada. J Clin Microbiol. 2004;42:5364–7. DOIPubMedGoogle Scholar
- Van Loo IH, Heuvelman KJ, King AJ, Mooi FR. Multilocus sequence typing of Bordetella pertussis based on surface protein genes. J Clin Microbiol. 2002;40:1994–2001. DOIPubMedGoogle Scholar
- Advani A, Donnelly D, Hallander H. Reference system for characterization of Bordetella pertussis pulsed-field gel electrophoresis profiles. J Clin Microbiol. 2004;42:2890–7. DOIPubMedGoogle Scholar
- Weber C, Boursaux-Eude C, Coralie G, Caro V, Guiso N. Polymorphism of Bordetella pertussis isolates circulating for the last 10 years in France, where a single effective whole-cell vaccine has been used for more than 30 years. J Clin Microbiol. 2001;39:4396–403. DOIPubMedGoogle Scholar
- Caro V, Elomaa A, Brun D, Mertsola J, He Q, Guiso N. Bordetella pertussis, Finland and France. Emerg Infect Dis. 2006;12:987–9.PubMedGoogle Scholar
- Packard ER, Parton R, Coote JG, Fry NK. Sequence variation and conservation in virulence-related genes of Bordetella pertussis isolates from the UK. J Med Microbiol. 2004;53:355–65. DOIPubMedGoogle Scholar
- Lin YC, Yao SM, Yan JJ, Chen YY, Hsiao MJ, Chou CY, Molecular epidemiology of Bordetella pertussis in Taiwan, 1993–2004: suggests one possible explanation for the outbreak of pertussis in 1997. Microbes Infect. 2006;8:2082–7. DOIPubMedGoogle Scholar
- Kodama A, Kamachi K, Horiuchi Y, Konda T, Arakawa Y. Antigenic divergence suggested by correlation between antigenic variation and pulsed-field gel electrophoresis profiles of Bordetella pertussis isolates in Japan. J Clin Microbiol. 2004;42:5453–7. DOIPubMedGoogle Scholar
- Guiso N, Boursaux-Eude C, Weber C, Hausman SZ, Sato H, Iwaki M, Analysis of Bordetella pertussis isolates collected in Japan before and after introduction of acellular pertussis vaccines. Vaccine. 2001;19:3248–52. DOIPubMedGoogle Scholar
- Han HJ, Kamachi K, Okada K, Toyoizumi-Ajisaka H, Sasaki Y, Arakawa Y. Antigenic variation in Bordetella pertussis isolates recovered from adults and children in Japan. Vaccine. 2008;26:1530–4. DOIPubMedGoogle Scholar
- Chen I, Finn TM, Yanqing L, Guoming Q, Rappuoli R, Pizza M. A recombinant live attenuated strain of Vibrio cholerae induces immunity against tetanus toxin and Bordetella pertussis tracheal colonization factor. Infect Immun. 1998;66:1648–53.PubMedGoogle Scholar
- Van Gent M, de Greeff SC, van der Heide HG, Mooi FR. An investigation into the cause of the 1983 whooping cough epidemic in the Netherlands. Vaccine. 2009;27:1898–903. DOIPubMedGoogle Scholar
- Van Gent M, Pierard D, Lauwers S, van der Heide HG, King AJ, Mooi FR. Characterization of Bordetella pertussis clinical isolates that do not express the tracheal colonization factor. FEMS Immunol Med Microbiol. 2007;51:149–54. DOIPubMedGoogle Scholar
- Hallander HO, Advani A, Donnelly D, Gustafsson L, Carlsson RM. Shifts of Bordetella pertussis variants in Sweden from 1970 to 2003, during three periods marked by different vaccination programs. J Clin Microbiol. 2005;43:2856–65. DOIPubMedGoogle Scholar
- Preston NW. Essential immunogens in human pertussis: the role of fimbriae. Dev Biol Stand. 1985;61:137–41.PubMedGoogle Scholar
- Mooi FR, van Loo IH, van Gent M, He Q, Bart MJ, Heuvelman KJ, Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg Infect Dis. 2009;15:1206–13. DOIPubMedGoogle Scholar
Figures
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 16, Number 11—November 2010
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Shumin Zhang, Department of Serum, National Institute for the Control of Pharmaceutical and Biological Products, Temple of Heaven, Beijing 100050, People’s Republic of China
Top