Volume 16, Number 4—April 2010
Dispatch
Novel Corynebacterium diphtheriae in Domestic Cats
Abstract
Novel nontoxigenic Corynebacterium diphtheriae was isolated from a domestic cat with severe otitis. Contact investigation and carrier study of human and animal contacts yielded 3 additional, identical isolates from cats, although no evidence of zoonotic transmission was identified. Molecular methods distinguished the feline isolates from known C. diphtheriae.
The clinical relevance of Corynebacterium diphtheriae recovered from a cat with otitis is poorly understood. Historically, humans have been thought to be its sole reservoir, and the few human cases reported annually in the United States are generally associated with international travel (1). Therefore, when C. diphtheriae was isolated from the ears of a cat, an investigation was initiated to evaluate potential sources of the cat’s infection and potential public health risks and to preliminarily characterize the C. diphtheriae isolate.
The cat, an 8-month-old female domestic shorthair, was examined at a West Virginia veterinary hospital on 5 occasions during January–June 2007. Pertinent findings included severe bilateral otitis, vestibular signs, mild ataxia, anorexia, and failure to gain weight; the cat had a history of ear, eye, and lung infections. Results of diagnostic tests showed no evidence of systemic disease and were negative for feline immunodeficiency and leukemia viruses and feline infectious peritonitis. Culture of an otic swab collected from the cat in May 2007 yielded 4 organisms: C. diphtheriae, Streptococcus equi zooepidemicus, Staphylococcus spp., and Achromobacter xylosoxidans. The cat was treated with oral clindamycin, otic enrofloxacin, and an ear-flushing solution.
In June 2007, investigators visited the veterinary clinic and the household of the index cat and conducted a contact investigation and carrier study. Interviews of 2 household members and 8 veterinary staff members indicated no recent respiratory illness, skin infection, or risk factors for diphtheria (e.g., travel to countries to which diphtheria is endemic or contact with known case-patients). Half of these 10 contacts had received diphtheria vaccination within the previous 5 years. Cultures of oropharyngeal swab samples obtained from each person were negative, including cystine tellurite blood agar, which is selective for C. diphtheriae. Household members also were interviewed about medical history of a convenience sample of household animals (4 cats, including the index cat; 2 dogs; and 1 horse). Each animal was briefly examined, and oropharyngeal, otic, or ocular swab samples were collected. Otitis was observed in all 4 cats and 1 dog. The horse reportedly had had an eye infection ≈5 years earlier. No other abnormal findings were noted. Animal specimens yielded 3 additional isolates of C. diphtheriae: 1 from each ear of the index cat and 1 from the left ear of a 2-year-old domestic medium-hair cat. Both cats had been born on the premises and had remained with the same household since birth.
Feline C. diphtheriae and reference isolates used are described in the Table. Tinsdale agar plate growth (Remel, Lenexa, KS, USA) gave rise to black colonies with a brown halo, typical of cysteinase-producing C. diphtheriae, C. ulcerans, or C. pseudotuberculosis. After 24 hours on blood agar, 1–2-mm grey-white or opaque, rounded, convex colonies with no hemolysis were observed. Microscopically, the bacteria were gram-positive, club-shaped rods, 1 µm in diameter, arranged singly or at angles. Biochemical profiles to determine species and biotype were done by using an API Coryne strip (bioMérieux, Durham, NC, USA, and St-Laurent, Quebec, Canada). Query of API Coryne code 0010304 obtained for all isolates by APIWEB (https://apiweb.biomerieux.com) indicated a decreased level of confidence of C. diphtheriae biotype mitis or belfanti (89.5%) because of a maltose-negative result. Isolates were further characterized morphologically and biochemically by using tube substrates (2) and were identified by using a standard taxonomic scheme (3). Feline isolates were biochemically identical with each other and phenotypically consistent with C. diphtheriae biotype belfanti, except for the lack of maltose fermentation, which was considered an unusual finding (3).
Antimicrobial drug susceptibility testing was performed according to the Clinical and Laboratory Standards Institute’s recommended methods and interpretative criteria (4). All 4 feline isolates were sensitive to ampicillin, cefepime, cefotaxime, ceftriaxone, cefuroxime, chloramphenicol, ciprofloxacin, clindamycin, daptomycin, erythromycin, ertapenam, gatifloxacin, gentamicin, levofloxacin, linezolid, meropenem, moxifloxacin, penicillin, quinupristin/dalfopristin, rifampin, telithromycin, tetracycline, tigecycline, trimethoprim/sulfamethoxazole, and vancomycin. Cellular fatty acid composition analysis was performed as described (5) by using the Sherlock system (MIDI, Inc., Newark, DE, USA), except that version 4.5 of the operating software was used. The cellular fatty acid composition profiles were consistent for C. diphtheriae, C. ulcerans, or C. pseudotuberculosis, including a substantial proportion (28%–30% of total) of C16:1ω7c (5). All feline isolates produced 7–15 meq/L of propionic acid among fermentation products, a feature associated with C. diphtheriae (2).
Results from use of the modified Elek test (6) indicated that all feline isolates were negative for production of diphtheria toxin; however, an atypical precipitation was observed after 36 h of incubation. Lack of toxin expression was corroborated by negative Vero cell assay results (7) and confirmed by using Western blot. Real-time PCR selective for the C. diphtheriae and C. ulcerans toxin gene (tox) (8) was positive for all feline isolates. However, real-time PCR for A and B subunits of tox (9) amplified subunit A but not subunit B. Sequence analysis of the tox gene was performed as previously outlined (10) and compared with a reference tox gene, GenBank accession no. K01722. The 4 feline tox sequences were identical to each other but contained multiple nucleotide substitutions and deletions compared with the reference gene. By NCBI BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi), the feline tox had higher sequence identity (97%–98%) to the tox sequences of C. ulcerans, compared with those from C. diphtheriae (94%–95%). A deletion at nt 55, coupled with a cytosine-to-thymine substitution at nt 74, prematurely terminated the peptide at aa 25.
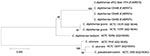
Species characterization was corroborated by using 16S rRNA (11) and partial rpoB (12) gene sequencing. By 16S rRNA gene sequence analysis, the feline strains had 100% identity with each other and >99.1% identity with various reference sequences for C. diphtheriae biotype gravis and belfanti sequences, including NCTC 11397T. Partial rpoB sequence analyses indicated 100% identity among the feline isolates and 97.7% identity with C. diphtheriae NCTC 11397T. Neighbor-joining phylogenetic trees based on both 16S rRNA (Figure 1) and partial rpoB gene sequencing (Figure 2) positioned the feline isolate sequences within the C. diphtheriae clade but clearly distinguished them from the other C. diphtheriae isolates. Comprehensive molecular analyses to characterize differences between biotype belfanti strains, including these feline isolates, with other C. diphtheriae biotypes, are the subject of a separate publication (C.G. Dowson, pers. comm.).
We identified a potentially novel biotype of C. diphtheriae recovered from domestic cats in West Virginia but found no evidence of zoonotic transmission. Although rare, isolation of C. diphtheriae from animals has been reported, including C. diphtheriae biotype belfanti from a skin lesion of a cow (13) and toxigenic C. diphtheriae biotype gravis from a wound of a horse (14). C. ulcerans is a known animal pathogen, and zoonotic transmission of toxigenic C. ulcerans from companion animals has been reported, often associated with predisposing concurrent illnesses (15).
The feline strains isolated during this investigation differed phenotypically from previously described biotypes but were otherwise regarded as typical of C. diphtheriae. However, isolates were nontoxigenic and harbored a modified tox gene with sequence differences from Corynebacterium spp. capable of expressing diphtheria toxin. On the basis of published criteria (11), the feline strain might represent a novel subspecies of C. diphtheriae because it shares <98% sequence homology to the type strain within the rpoB gene. Potential for zoonotic transmission of this novel, cat-associated C. diphtheriae and associated public health implications are unknown. Additional studies are needed to further characterize these isolates and determine their appropriate taxonomy. Large-scale screening of domestic cat populations is recommended to determine the prevalence of C. diphtheriae and its pathogenic potential and to identify additional isolates for more formal description and classification.
Dr Hall is a public health veterinarian who completed this study while serving as an Epidemic Intelligence Service Officer of the Centers for Disease Control and Prevention (CDC), assigned to the state of West Virginia. Currently he is an epidemiologist on the CDC Viral Gastroenteritis Team. His research interests focus on public health issues involving interactions between humans, domestic animals, wildlife, and the environment.
Acknowledgments
We gratefully recognize Amy Isaac, Gary Kinder, and Katrina Kretsinger for collaborative assistance in the epidemiologic investigation and Tamara Burdz, Christi Clark, Tiffany Jackson, Brenda Keavey, Betty Ng, Chris Paddock, and Deborah Wiebe for laboratory assistance.
Work performed at University of Warwick was funded in part by the Biotechnology and Biological Sciences Research Council, Micropathology Ltd, and the Medical Research Fund.
References
- Vitek CR, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia: Elsiever; 2008. p. 139–56.
- Bernard KA, Munro C, Wiebe D, Ongsansoy E. Characteristics of rare or recently described Corynebacterium species recovered from human clinical material in Canada. J Clin Microbiol. 2002;40:4375–81. DOIPubMedGoogle Scholar
- Funke G, Bernard KA. Coryneform gram-positive rods. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Washington: ASM Press; 2007. p. 485–514.
- Clinical Laboratory Standards Institute. M45-A. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. Wayne (PA): The Institute; 2006.
- Bernard KA, Bellefeuille M, Ewan EP. Cellular fatty acid composition as an adjunct to the identification of asporogenous, aerobic gram-positive rods. J Clin Microbiol. 1991;29:83–9.PubMedGoogle Scholar
- Engler KH, Glushkevich T, Mazurova IK, George RC, Efstratiou A. A modified Elek test for detection of toxigenic corynebacteria in the diagnostic laboratory. J Clin Microbiol. 1997;35:495–8.PubMedGoogle Scholar
- Miyamura K, Nishio S, Ito A, Murata R, Kono R. Micro cell culture method for determination of diphtheria toxin and antitoxin titres using VERO cells. I. Studies on factors affecting the toxin and antitoxin titration. J Biol Stand. 1974;2:189–201. DOIPubMedGoogle Scholar
- Schuhegger R, Lindermayer M, Kugler R, Heesemann J, Busch U, Sing A. Detection of toxigenic Corynebacterium diphtheriae and Corynebacterium ulcerans strains by a novel real-time PCR. J Clin Microbiol. 2008;46:2822–3. DOIPubMedGoogle Scholar
- Mothershed EA, Cassiday PK, Pierson K, Mayer LW, Popovic T. Development of a real-time fluorescence PCR assay for rapid detection of the diphtheria toxin gene. J Clin Microbiol. 2002;40:4713–9. DOIPubMedGoogle Scholar
- Cassiday PK, Pawloski LC, Tiwari T, Sanden GN, Wilkins PP. Analysis of toxigenic Corynebacterium ulcerans strains revealing potential for false-negative real-time PCR results. J Clin Microbiol. 2008;46:331–3. DOIPubMedGoogle Scholar
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703.PubMedGoogle Scholar
- Khamis A, Raoult D, La Scola B. rpoB gene sequencing for identification of Corynebacterium species. J Clin Microbiol. 2004;42:3925–31. DOIPubMedGoogle Scholar
- Corboz L, Thoma R, Braun U, Zbinden R. Isolation of Corynebacterium diphtheriae subsp. belfanti from a cow with chronic active dermatitis [in German]. Schweiz Arch Tierheilkd. 1996;138:596–9.PubMedGoogle Scholar
- Henricson B, Segarra M, Garvin J, Burns J, Jenkins S, Kim C, Toxigenic Corynebacterium diphtheriae associated with an equine wound infection. J Vet Diagn Invest. 2000;12:253–7.PubMedGoogle Scholar
- Bonmarin I, Guiso N, Le Fleche-Mateos A, Patey O, Patrick AD, Levy-Bruhl D. Diphtheria: a zoonotic disease in France? Vaccine. 2009;27:4196–200. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 16, Number 4—April 2010
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Aron J. Hall, Division of Viral Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop A47, Atlanta, GA 30333, USA
Top