Volume 16, Number 6—June 2010
Research
Increased Prevalence of Trichinella spp., Northeastern Germany, 2008
Cite This Article
Citation for Media
Abstract
In 2008, a Trichinella spp. outbreak occurred on a small family-owned pig farm in Mecklenburg–Western Pomerania in northeastern Germany. To obtain epidemiologic information on this outbreak, we determined that after 2005 the prevalence of Trichinella spp. in wild boars has increased in this region of Germany. We discuss the potential role of the raccoon dog in the increase in Trichinella spp. prevalence in the sylvatic cycle in this region. We believe that this increase could pose a threat to pigs kept in back yard conditions, and we provide recommendations to ensure public health safety.
Nematodes of the genus Trichinella infect a broad variety of mammals, birds, and reptiles and are distributed worldwide (1). Trichinellosis is a foodborne zoonotic disease caused by a parasite. Human infections occur after ingestion of raw or inadequately cooked meat containing parasite larvae (2). Pigs represent a major source of human infection, but meat from horses, wild boars, bears, and badgers have also played a major role during outbreaks (3,4).
Trichinella spp. can be transmitted by domestic and sylvatic cycles. The domestic cycle is maintained by feeding of swill to pigs and pigs feeding on animal carcasses or on synanthropic animals (e.g., rats, mice). In Germany, the domestic cycle disappeared >30 years ago (5). During 1998–2007, ≈436 million pigs were slaughtered and tested for Trichinella spp. by using artificial digestion according to regulation (European Commission [EC]) no. 2075/2005 (6). However, in 2003, 1 positive case was reported; it was in a pig kept in a back yard (7).
Currently, the major Trichinella spp. reservoir and source of infection for domestic pigs in Germany is wild boars (Sus scrofa), raccoon dogs (Nyctereutes procyonoides), and foxes (Vulpes vulpes). The most prevalent Trichinella spp. is T. spiralis, followed by T. britovi and T. pseudospiralis (8).
In the past 30 years, sporadic human infections with Trichinella spp. (0–50 reported cases per year) have occurred in Germany (7,9). These infections are usually linked to consumption of contaminated meat during holiday visits to high-risk countries (10). Autochthonous outbreaks occur infrequently, such as during 2005–2006, when 17 members of a large family in Mecklenburg–Western Pomerania were infected with Trichinella spp. after consumption of meat products from a pig reared and slaughtered at home (11).
We report a Trichinella spp. outbreak on a small family-owned pig farm in Mecklenburg–Western Pomerania in northeastern Germany during December 2008. We show that after 2005, the prevalence of Trichinella spp. in wild boars has increased in this region of Germany. Furthermore, we discuss the possibility that increased Trichinella spp. prevalence in wild boars is the result of high prevalence of the disease in neighboring Poland. The potential role of migration of raccoon dogs from Poland into Germany is also considered as a factor of increased prevalence.
Outbreak Investigations
Veterinary inspections of the outbreak farm were performed with emphasis on production type, location, potential contacts with wild animals (including rodents), feeding habits, Trichinella spp. status of animals on origin farms, and human factors. Meat and blood samples of 5 slaughtered pigs from the outbreak farm were sent to the National Reference Laboratory for Trichinellosis at the Federal Institute for Risk Assessment in Berlin, Germany.
Laboratory Examinations
Muscle Samples
All muscle samples from Germany and Poland were examined by artificial digestion (magnetic stirrer method) according to regulation (EC) no. 2075/2005 (6). A mouse caught in a trap on the outbreak farm was examined by using the same method.
PCR
Larvae were isolated from muscle tissues and washed 4× with distilled water on ice and stored in 5 µL of distilled water at –20°C. DNA extraction and PCR were performed as described by Pozio and La Rosa (12).
Serologic Testing of Blood and Meat Juice
Venous blood was collected from live pigs. Meat was obtained from the diaphragm pillar of slaughtered pigs, cut in small pieces, put in plastic bags, and frozen at –20°C for 3 days. After thawing, the meat was squeezed and meat juice was collected and stored at –20°C. Serum samples were examined by using an in-house ELISA as described by Nöckler et al. (13). This assay is based on the excretory–secretory antigen (14).
Sampling, Data Management, and Statistical Analysis
There are currently no reliable estimates of the number of wild boars in Germany. However, the number of wild boars hunted during a hunting year in a specific region is considered proportional to the size of the wild boar population (15). To optimally control the wild boar population and prevent damage to land or crops and the spread of disease, wild boars in Germany are hunted annually (16). Depending on climate, reproduction rate, and hunting success, ≈50%–100% of the animals born in a given year are hunted (killed) in Germany each year (17). According to specifications of German hunting associations, the number of wild boars hunted should consist of 80% piglets (maximum age 1 year), 10% juveniles (1–2 years of age), and 10% older sows. However, because hunters generally focus on older piglets and juveniles, the suggested number of wild boars hunted is not representative of the population and is biased toward younger animals. This factor could lead to an underestimation of the Trichinella spp. prevalence in wild boars because young animals only have a short time span during which they can become infected.
The number of foxes and raccoon dogs hunted is also thought to reflect changes in the sizes of the populations of these species. Raccoon dogs and foxes are not examined for Trichinella spp. according to regulation (EC) no. 2075/2005, and currently, Germany has no countrywide monitoring program for Trichinella spp. in these animals. Animals examined were part of research initiatives. Therefore, sampling of raccoon dogs and foxes is less representative than that of wild boars. Information on wild boars examined each year for Trichinella spp. in the German Federal States during 2002–2008 was supplied by the German Federal Statistics Office (18).
During 2002–2008, prevalences of Trichinella spp.–positive wild boars in Ostvorpommern, Germany, were compared with those in Mecklenburg–Western Pomerania and in the remaining German Federal States. Data for Trichinella spp. prevalences in foxes and raccoon dogs hunted in Mecklenburg–Western Pomerania during 1993–2008 and in wild boars and foxes hunted during 2004–2008 on Island Wolin, Poland, which borders Ostvorpommern, were also analyzed. We determined 95% confidence intervals from the binomial distribution as described by Clopper and Pearson (19). Descriptive statistics were calculated by using available online resources (www.statpages.org).
Outbreak Investigations
In November 2008, an 11-month-old pig (German Landrace × Large White) was declared positive for Trichinella spp. at an abattoir in Ostvorpommern, a district in Mecklenburg–Western Pomerania. The day after detection of Trichinella larvae, veterinarians of the Veterinary and Food Safety Authority visited the outbreak farm and conducted epidemiologic investigations. A blood sample obtained for swine fever monitoring shortly before slaughtering was sent to the National Reference Laboratory for Trichinellosis. The remaining 4 pigs at this site were slaughtered ≈2 weeks after the first case of Trichinella infection was identified.
Description of Farm
The farm was a small holding of ≈0.5 acres at the edge of a village bordering a large field. In addition to the 5 pigs, sheep, poultry, cats, and a dog also lived on the farm. The pigs were penned in an old, closed building and allowed to enter a small outdoor yard once a week. The farm was run in accordance with the German Pig-Keeping Hygiene Ordinance.
Rodent Manifestations
During the summer of 2008, the farmer observed some rats on the premises. A professional pest control company was contacted after the slaughtering of all pigs, but no rodent infestation was detected.
Feeding
The pigs were routinely fed steamed potatoes, potato ensilage, fodder beets, feed grain, sugar beet pellets, and water. According to the farmer, the pigs were not fed swill.
Trichinella spp. Status of Origin Farm
At the beginning of 2008, a total of 3 Trichinella spp.–positive animals had been bought as weaned pigs from a small breeding farm, which had 8 breeding sows, 2 boars, and ≈60 piglets ≈3 km from the outbreak farm. Blood samples were taken from 16 animals (including 8 breeding sows) for testing by ELISA. All pigs sold from and slaughtered at this breeding farm in 2008 were traced and confirmed to have been negative for Trichinella spp.
The 2 Trichinella spp.–negative pigs from the outbreak farm were obtained as finishers from a large commercial fattening unit that delivered hundreds of finished pigs per week to an EU-licensed abattoir. To date, all pigs from the commercial piggery have been negative for Trichinella spp.
Human Factors
Until the fall of 2008, a family member living on the outbreak farm was employed as a cook in a restaurant near the border with Poland. The restaurant is well known for game dishes and has its own meat-cutting room and area for selling meat. During an inspection of the restaurant, veterinarians found evidence that meat offal was being supplied to unknown owners of animals. However, additional information (number or identity of owners of animals) could not be obtained.
Laboratory Examinations
Muscle Examination
One Trichinella spp.–positive pig slaughtered had 299 larvae/g of tissue, and the 2 other pigs kept in the area had 1.2 and 1.3 larvae/g of tissue, respectively (Table 1). The other 2 pigs kept in a separate area on the farm were negative for Trichinella spp. Larvae from all 3 Trichinella spp.–positive pigs were examined by PCR and identified as T. spiralis. All 3 Trichinella spp.–positive carcasses were classified as unfit for human consumption.
Serologic Examination
ELISAs were conducted for blood and meat juice samples from the 5 pigs on the outbreak farm. Results were consistent with those of the magnetic stirrer method (Table 1). All 16 blood samples from the 3 Trichinella spp.–positive pigs from the origin farm were negative for Trichinella spp. by ELISA. Antibodies against Trichinella spp. were not detected in the serum of the farmer (immunoglobulin G titer <10, immunoglobulin M titer <40).
Prevalence of Trichinella spp. in Wild Boars in Germany, 2002–2008
In Germany, examination of wild boar carcasses intended for human consumption is compulsory (6). Because reporting to the German Federal Statistical Office is obligatory, the number of wild boars examined per year was considered a representative sampling unit.
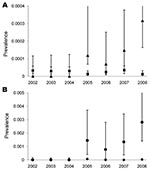
The yearly prevalence of Trichinella spp. in wild boars in Germany during 2002–2008 ranged from 0.0027% to 0.0032% (Table 2). In 2005, there was a sudden increase in Trichinella spp. prevalence in wild boars in Mecklenburg–Western Pomerania compared with the rest of Germany. During 2005 and 2008 (Figure 1, panel A) and during 2005–2008, more Trichinella spp.–positive wild boars were detected in Mecklenburg–Western Pomerania than in the rest of Germany.
Within Mecklenburg–Western Pomerania, 21 (87.5%) of 24 Trichinella spp.–positive results were in wild boars in Ostvorpommern, the district where the outbreak farm was located. Trichinella spp. prevalence in Oatvorpommern was higher in 2005, 2007, and 2008 than in the rest of Mecklenburg–Western Pomerania (Figure 1, panel B).
For verification and species identification, 20 Trichinella spp.–positive wild boar samples obtained during 2005–2008 were sent to the National Reference Center for Trichinellosis. A total of 80% (14) were identified as T. spiralis, 15% (3) as T. pseudospiralis, and 1 as a mixed infection (T. spiralis and T. pseudospiralis) (20). Larval load ranged from 2 to 922 larvae/g of tissue.
Trichinella spp.–Positive Raccoon Dogs and Foxes, Mecklenburg–Western Pomerania
The number of raccoon dogs and red foxes hunted in Mecklenburg–Western Pomerania is shown in Table 3. During 1993–2008, a sharp increase in raccoon dogs hunted was observed; the number of foxes hunted remained constant over this period. No difference was found between the number of foxes and raccoon dogs hunted in Ostvorpommern and those hunted in more westward districts of similar sizes in Mecklenburg–Western Pomerania.
In Germany, raccoon dogs and foxes are not routinely examined for Trichinella spp. In a monitoring program conducted in Mecklenburg–Western Pomerania during February 2006–January 2007, a total of 100 raccoon dogs and foxes were examined by using the magnetic stirrer method. Trichinella spp. prevalence was 4.0% in raccoon dogs and 1.0% in foxes. In a smaller district-level monitoring study during February–August 2006, a total of 3 of 46 raccoon dogs from Ostvorpommern and a neighboring district were positive for Trichinella spp. (prevalence 6.5%). Larval load ranged from 0.06 to 65 larvae/g of tissue. Four of 7 raccoon dogs were infected with T. spiralis and 2 with T. pseudospiralis. One raccoon dog from Ostvorpommern had a mixed infection (T. spiralis and T. pseudospiralis).
Trichinella spp. Prevalence in Wild Boars and Raccoon Dogs, Ostvorpommern
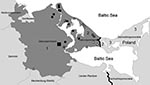
Ostvorpommern is located in the eastern part of Mecklenburg–Western Pomerania, Germany (Figure 2). Part of this district is on Usedom Island, an island in the Baltic Sea. The western part of Usedom Island is in Germany and the eastern part is in Poland. Of the 26 reported Trichinella spp. infections in wild animals (21 in wild boars and 5 in raccoon dogs) in Ostvorpommern during 2005–2008, a total of 80.7% were found on Usedom Island (Figure 2).
Trichinella spp. Prevalence in Wild Boars and Foxes, Wolin Island, Poland, 2004–2008
On Wolin Island, which borders Usedom Island, 22 (3.27%) of 672 wild boars examined during 2004–2008 were positive for Trichinella spp. During the same period, 6 (4.22%) of 142 foxes were positive for Trichinella spp. (Table 4).
In December 2008 a Trichinella spp. outbreak occurred on a pig farm in Mecklenburg–Western Pomerania, Germany. Although the affected animals originated from a farm with similar biosecurity levels as the outbreak farm, negative serologic results for pigs at the origin farm and the longer period spent on the outbreak farm make infection on the outbreak farm more likely.
Potential infection sources were manifold. Although trichinellosis is not a contagious disease, all pigs kept in 1 area were positive for Trichinella spp. This finding suggests a common source of infection. At the time of outbreak investigations in December 2008, no evidence of rodents was found on the farm. However, the role of rats as a Trichinella spp. maintenance host is unclear, and infection in rats is considered a marker for infection in pigs (21).
Feeding of infectious meat (e.g., wild boar) can also lead to Trichinella spp. infection in pigs. The farmer on the outbreak farm had access to game meat because a family member worked at a restaurant in which game meat offal was given to owners of animals. Although the direct circumstances could not be elucidated, game meat offal seems to be the most likely source of infection.
The yearly prevalence of Trichinella spp. in wild boars in Germany ranged from 0.0027% in 2002 to 0.0045% in 2008. In contrast, during 1975–2005, Trichinella spp. were not detected in wild boars in Mecklenburg–Western Pomerania. Since 2005, Trichinella spp. prevalence has increased in Mecklenburg–Western Pomerania in wild boars in comparison with the rest of Germany. Mecklenburg–Western Pomerania, the district in which Ostvorpommern is located, has been predominantly affected. A total of 80.7% of all wild boars and raccoon dogs positive for Trichinella spp. were found on Usedom Island.
Since 2005, the trichinoscopic method for larvae detection has been replaced by more sensitive methods of artificial digestion (6). Because new legislation regarding trichinellosis was implemented at the same time in Germany, it is unlikely that higher Trichinella spp. prevalence in wild boars in Mecklenburg–Western Pomerania can be attributed to the use of this improved diagnostic technique.
Human trichinellosis is regularly reported in Poland. Thirty-five human outbreaks and 702 cases were reported in Poland during 2002–2007 (22). Because of stringent control measures in recent years, the primary source of human infection has changed from pork to wild boar meat (23). During 1999–2004, Balicka-Ramisz et al. (24) examined >56,000 wild boars in West Pomerania, a province in northwestern Poland bordering Mecklenburg–Western Pomerania, Germany. Trichinella spp. prevalence in wild boars in this region increased 8-fold from 0.12% to 1.48% during this period. In the same study, >500 foxes from West Pomerania were tested; Trichinella spp. prevalence was 4.4%. Trichinella spp. prevalence in wild boars on Wolin Island Poland was even higher (6.12% in 2008).
In Germany, the Trichinella spp. prevalence in foxes is <1% (25–27). However, the raccoon dog can also play a major role in maintenance of the Trichinella spp. sylvatic cycle (28). Raccoon dogs were first reported in Poland in 1955 (29); this species has become established in eastern Europe and has extended its settlement area to the west and south. The raccoon dog population in Germany has increased dramatically in recent years. In the former German Democratic Republic, only 58 raccoon dogs were hunted before 1987 (30). In 2001–2002, a total of ≈12,000 raccoon dogs were hunted in Germany; >96.0% were hunted in Mecklenburg–Western Pomerania and Brandenburg, the most eastern German Federal States (31). Studies in eastern Germany showed that 25.9%–35.1% of the raccoon dog diet is carrion (32). When intensity of infection in these animals was compared with that of other carnivores, the raccoon dog had higher larvae loads (33,34), which indicates the role of this species as a Trichinella spp. reservoir. In addition, T. spiralis can survive in rat carcasses during the summer in northern Europe for 2–4 weeks (33). Thiess et al. (28) examined 120 raccoon dogs from the northern part of Brandenburg; T. spiralis was identified in 5.0%. Results of that study support our findings in Mecklenburg–Western Pomerania.
Our data are insufficient to fully ascertain risk factors specific for the increase in Trichinella spp. prevalence in this region. Although for some years the raccoon dog has become widespread in Mecklenburg–Western Pomerania and Brandenburg in recent years, the Trichinella spp. prevalence in wild boars has increased mainly on Usedom Island. As for wild boars, the number of raccoon dogs hunted is considered to be proportional to the size of the total population. During 2005–2008, ≈34,000 raccoon dogs (1.15 raccoon dogs/km2) were hunted in Brandenburg. During the same period, 3.38 raccoon dogs/km2 were hunted in Mecklenburg–Western Pomerania, which indicated that the raccoon dog population in this region is larger than that in Brandenburg (35–38). During this time, numbers of wild boars and foxes did not differ between these 2 regions of Germany, and the hunting activity was expected to be similar in both regions.
The optimal habitat for raccoon dogs is wet areas and fields, small forests, and large ditches bordered by thick vegetation (39); this habitat corresponds to the landscape in northeastern Germany and northwestern Poland. Also, since 1960, large areas of the Wolin Island have been designated as a national park, which may have led to an increase in the raccoon dog population. Because the body of water separating Wolin Island from Usedom Island is narrower than the Stettiner Haff Lagoon and the Untere Oder (part of the Oder River) to the south, raccoon dog migration from east to west might have occurred through this route. Whether future increased spread of raccoon dogs in Brandenburg will result in increased Trichinella spp. prevalence in wild boars is unclear.
In summary, since 2005, an increase in Trichinella prevalence spp. in wild boars has occurred in Mecklenburg–Western Pomerania, Germany. This increase in Trichinella spp. prevalence in the sylvatic cycle may be associated with spread of raccoon dogs in this region. This increase poses a threat to public health because of pigs kept in backyards. Backyard farming is fairly common in the region and because of economic reasons has been traditionally associated with feeding of kitchen scraps to pigs.
For intensive pig production units that practice high levels of biosecurity, risk for Trichinella spp. infection is low. Although feeding with swill is illegal (40), feeding of kitchen scraps including wild boar offal still occurs, which increases risk for human outbreaks. Although scavenging of wild boar, fox, or raccoon dog carcasses by pigs is rare, exposure of domestic pigs kept outdoors to Trichinella spp. cannot be excluded. Therefore, farmers should be made aware of increased potential risk factors, especially feeding with swill. Also, hunters should be encouraged to remove carcasses from the forest or bury them at an appropriate depth. It would also be advisable to monitor whether the western and southern migration of raccoon dogs is indicative of increased Trichinella spp. prevalence in the sylvatic cycle in newly settled areas. Furthermore, programs are needed that emphasize the necessity of ensuring testing for Trichinella spp. infection in all wild boars intended for human consumption and promoting education of humans regarding thorough cooking of meat to guarantee food safety.
Dr Pannwitz is district veterinary officer in Ostvorpommern, Mecklenburg–Western Pomerania. His main interests include zoonoses, veterinary epidemiology, and veterinary public health.
Acknowledgment
We thank Sascha Gerst for providing data on foxes and raccoon dogs; Nicole Awe, Holger Vogel, Heike Kerlikowsky, and Gaby Splittgerber for providing hunting statistics and data on wild boars; and Sabine Reckinger for performing serologic, parasitologic, and molecular examinations.
References
- Gottstein B, Pozio E, Nockler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22:127–42. DOIPubMedGoogle Scholar
- Dorny P, Praet N, Deckers N, Gabriel S. Emerging food-borne parasites. Vet Parasitol. 2009;163:196–206. DOIPubMedGoogle Scholar
- Ozeretskovskaya NN, Mikhailova LG, Sabgaida TP, Dovgalev AS. New trends and clinical patterns of human trichinellosis in Russia at the beginning of the XXI century. Vet Parasitol. 2005;132:167–71. DOIPubMedGoogle Scholar
- Boireau P, Vallee I, Roman T, Perret C, Mingyuan L, Gamble HR, Trichinella in horses: a low frequency infection with high human risk. Vet Parasitol. 2000;93:309–20. DOIPubMedGoogle Scholar
- Pozio E, Hoberg E, La Rosa G, Zarlenga DS. Molecular taxonomy, phylogeny and biogeography of nematodes belonging to the Trichinella genus. Infect Genet Evol. 2009;9:606–16. DOIPubMedGoogle Scholar
- Commission Regulation (EC) no. 2075/2005 of 5 December 2005. Laying down specific rules on official controls for Trichinella in meat [cited 2010 Mar 21]. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:338:0060:0082:EN:PDF
- Nöckler K. Vorkommen und bedeutung von Trichinella spp. in Deutschland. Veterinary Medicine Austria. 2005;92:301–7.
- International Trichinella Reference Center [cited 2010 Feb 10]. http://www.iss.it/site/Trichinella/index.asp
- Robert Koch Institute. SurvStat@RKI [cited 2009 Jan 1]. http://www3.rki.de/SurvStat/
- Jansen A, Schöneberg I, Stark K, Nöckler K. Epidemiology of trichinellosis in Germany, 1996–2006. Vector Borne Zoonotic Dis. 2008;8:189–96. DOIPubMedGoogle Scholar
- Littmann M, Nöckler K. Trichinellose: Zu einer Häufung in Mecklenburg-Vorpommern. [RKI]. Epidemiologisches Bulletin. 2006;18:139–40.
- Pozio E, La Rosa G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol Biol. 2003;216:299–309.PubMedGoogle Scholar
- Nöckler K, Voigt WP, Protz D, Miko A, Ziedler K. Diagnosis of trichinellosis in living pigs using indirect ELISA [in German]. Berl Munch Tierarztl Wochenschr. 1995;108:167–74.PubMedGoogle Scholar
- Gamble HR, Anderson WR, Graham CE, Murrell KD. Diagnosis of swine trichinosis by enzyme-linked immunosorbent assay (ELISA) using an excretory–secretory antigen. Vet Parasitol. 1983;13:349–61. DOIPubMedGoogle Scholar
- Leuenberger R, Boujon P, Thür B, Miserez R, Garin-Bastuji B, Rüfenacht J, Surveillance of wild boar in Switzerland: prevalence of classical swine fever, Aujeszky's disease and brucellosis. Vet Rec. 2007;160:362–8. DOIPubMedGoogle Scholar
- Praxishilfe Wildschweinmanagement. Schweizerische eidgenossenschaft, bundesamt für umwelt BAFU. Teil A [cited 2010 Feb 10]. http://www.wildschwein-sanglier.ch/ws_start.php?la=d&th=prax&st=1
- Ökologischer Jagdverband Sachsen [cited 2010 Feb 10]. http://www.sachsen-oejv.de/index.html
- German Federal Statistical Office. Fachserie 3 Reihe 4.3 (2001–2007) [cited 2010 Mar 21]. http://www.destatis.de/jetspeed/portal/cms/Sites/destatis/Internet/EN/press/pr/2008/01/PE08__015__811,templateId=renderPrint.psml
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. DOIGoogle Scholar
- Nöckler K, Reckinger S, Pozio E. Trichinella spiralis and Trichinella pseudospiralis mixed infection in a wild boar (Sus scrofa) of Germany. Vet Parasitol. 2006;137:364–8. DOIPubMedGoogle Scholar
- Pozio E, Zarlenga DS. Recent advances on the taxonomy, systematics and epidemiology of Trichinella. Int J Parasitol. 2005;35:1191–204. DOIPubMedGoogle Scholar
- Sadkowska-Todys M, Golab E, Rosinska M. Geographical distribution of human and animal trichinellosis in Poland: is there a connection? 5th Annual Scientific Meeting of MedVetNet, 2009 May 5; Madrid, Spain. Maisons-Alfort (France): Agence Française de Sécurité Sanitaire des Aliments. Abstract book; 2009. p. 32.
- Golab E, Sadkowska-Todys M. Epidemiology of human trichinellosis in Poland—currently and in the past [in Polish]. Wiad Parazytol. 2006;52:181–7.PubMedGoogle Scholar
- Balicka-Ramisz A, Grupinski T, Ramisz A, Pilarczyk B, Laurans L. Prevalence of Trichinella spp. in red foxes and wild boars in the northwestern part of Poland [in German]. Dtsch Tierarztl Wochenschr. 2007;114:354–7.PubMedGoogle Scholar
- Wacker K, Rodriguez E, Garate T, Geue L, Tackmann K, Selhorst T, Epidemiological analysis of Trichinella spiralis infections of foxes in Brandenburg, Germany. Epidemiol Infect. 1999;123:139–47. DOIPubMedGoogle Scholar
- Wagner JA, Frank W. Recent research on the prevalence of Trichinella in wild mammals in the Federal Republic of Germany. In: Tanner CE, Martinez-Fernandez AR, Bolas-Fernandez, F, editors. Trichinellosis. Proceedings of the Seventh International Conference on Trichinellosis, 1988 May 2–6; Alicante, Spain. Madrid: CSIC Press; 1989. p. 416–22.
- Hoffmann L, Klengel K, Worbes H, Orthey G. Zur Prävalenz von Echinococcus multilocularis und Trichinella spp. beim Rotfuchs in Thüringen. Neue Methoden und Ergebnisse zur Epidemiologie von Parasitosen. Hannover (Germany): DVG Tagung, 10–12.03; 1999.
- Thiess A, Schuster R, Nöckler K, Mix H. Helminth findings in indigenous raccoon dogs Nyctereutes procyonoides (Gray, 1843) [in German]. Berl Munch Tierarztl Wochenschr. 2001;114:273–6.PubMedGoogle Scholar
- Dudzinski W, Haber A, Matuszewski G. Die Verbreitung des Marderhundes (Nyctereutes procyonoides Gray) in Polen. Z Jagdwiss. 1963;9:98. DOIGoogle Scholar
- Stubbe M. Neue Erkenntnisse zur Ökologie und Verbreitung der Marderhundes Nyctereutes procyonoides (Gray, 1834) in der DDR. Beiträage zur Jagd- und Wildforschung. 1989;16:261–7.
- Goretzki J. Invasoren auf vier pfoten. Forschungsreport. 2003;2:24–7.
- Thiess A. Untersuchungen zur Helminthenfauna und zum Vorkommen von Trichinella sp. beim Marderhund (Nyctereutes procyonoides) in Brandenburg [dissertation]. Berlin: Federal University; 2004.
- Oivanen L, Kapel CMO, Pozio E, La Rosa G, Mikkonen T, Sukura A. Associations between Trichinella species and host species in Finland. J Parasitol. 2002;88:84–8.PubMedGoogle Scholar
- Malakauskas A, Paulauskas V, Jarvis T, Keidans P, Eddi C, Kapel CM. Molecular epidemiology of Trichinella spp. in three Baltic countries: Lithuania, Latvia and Estonia. Parasitol Res. 2007;100:687–93. DOIPubMedGoogle Scholar
- Jagdbericht Mecklenburg-Vorpommern 2006/07 [cited 2009 Apr 17]. http://www.ljv-mecklenburg-vorpommern.de/Jagdbericht%202006-2007.pdf
- Jagdbericht Mecklenburg-Vorpommern 2005/06 [cited 2009 Apr 17]. http://www.ljv-mecklenburg-vorpommern.de/Jagdbericht_2005_2006.pdf
- Jagdbericht Mecklenburg-Vorpommern 2004/2005 [cited 2009 Apr 17]. http://www.kora.ch/malme/05_library/5_1_publications/R/Rackwitz_et_al_2006_13.Jagdbericht_Mecklenburg_Vorpommern.pdf
- Jagdberichte des Landes Brandenburg [cited 2009 Apr 17]. http://www.mluv.brandenburg.de/cms/detail.php/124304
- Holmala K, Kauhala K. Ecology of wildlife rabies in Europe. Mammal Rev. 2006;36:17–36. DOIGoogle Scholar
- Regulation (EC) no. 1774/2002 of the European Parliament and of the Council of 3 October 2002. Laying down health rules concerning animal products not intended for human consumption [cited 2010 Mar 21]. http://europa.eu/legislation_summaries/food_safety/specific_themes/f81001_en.htm
Figures
Tables
Cite This ArticleTable of Contents – Volume 16, Number 6—June 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Anne Mayer-Scholl, Federal Institute for Risk Assessment, Diedersdorfer Weg 1, Berlin 12277, Germany
Top