Volume 17, Number 2—February 2011
Research
Phocine Distemper Virus in Seals, East Coast, United States, 2006
Cite This Article
Citation for Media
Abstract
In 2006 and 2007, elevated numbers of deaths among seals, constituting an unusual mortality event, occurred off the coasts of Maine and Massachusetts, United States. We isolated a virus from seal tissue and confirmed it as phocine distemper virus (PDV). We compared the viral hemagglutinin, phosphoprotein, and fusion (F) and matrix (M) protein gene sequences with those of viruses from the 1988 and 2002 PDV epizootics. The virus showed highest similarity with a PDV 1988 Netherlands virus, which raises the possibility that the 2006 isolate from the United States might have emerged independently from 2002 PDVs and that multiple lineages of PDV might be circulating among enzootically infected North American seals. Evidence from comparison of sequences derived from different tissues suggested that mutations in the F and M genes occur in brain tissue that are not present in lung, liver, or blood, which suggests virus persistence in the central nervous system.
In 1988, harbor seals (Phoca vitulina) and gray seals (Halichoerus grypus) died in large numbers off the coast of northern Europe (1). A virus was first isolated in April 1988, when widespread abortions and deaths among harbor seals were reported in the Kattegat area between Denmark and Sweden. The infection spread to the North, Wadden, and Baltic seas, killing 17,000–20,000 seals in northwestern Europe in 8 months. The virus subsequently was classified as a species of the genus Morbillivirus (family Paramyxoviridae) (2,3), Phocine distemper virus (PDV). The virus is believed to have originated in harp seals in which the infection is enzootic (4). Migrations of harp seals into the North Sea may have initiated the epizootic in harbor seals. Gray seals in the northeastern Atlantic Ocean also were infected, but disease was not as severe as in harbor seals (5).
A more recent outbreak occurred in Europe in 2002 (6). An estimated 30,000 harbor and gray seals died during this epizootic (7,8). The origin of this second epizootic 14 years after the first remains unknown. PDV may have jumped species into terrestrial carnivores, particularly mink, and reinfected seals (9), but this hypothesis remains unproven. Phylogenetic analysis of the hemagglutin (H) genes of PDV, together with those of other morbilliviruses, suggests that the reemergent 2002 PDV is more closely related to a putative recent ancestral PDV than to the 1988 isolates (10). Millions of seals of various species inhabit the waters surrounding North America; populations of most species are believed to be stable or increasing, and no epizootics on the scale of those reported in Europe have been reported. PDV disease in the United States was first reported in harbor seals on the east coast during the winter of 1991–92 (11), and serologic testing of gray and harbor seals suggested that a PDV-like strain or strains were circulating enzootically in the region (12). This circulation was attributed to an increased number of harbor seals (mainly immature animals) overwintering in southern New England (13). During the spring of 2006, deaths among seals (harbor, gray, and hooded) also increased along the coasts of Maine and Massachusetts. This increase was considered an unusual mortality event. Both dead and sick seals appeared nonemaciated. Live-stranded seals were weak and had generalized body tremors and spasms. Affected seals were taken to the Marine Science Education and Research Center (University of New England, Biddeford, ME, USA); investigations indicated that the pathologic changes were consistent with morbillivirus infection. Recent advances in virus isolation and genetic sequencing methods have provided us with better insight into PDV epizootiology in Europe and in North America.
We isolated the 2006 virus from liver tissue of a harbor seal and confirmed it as PDV. To determine the phylogenetic relationship and possible origin of the isolate, we compared the virus RNA sequences and deduced amino acid sequences for the virus cell receptor attachment protein hemagglutinin (H) with those from various PDVs from both the 1988 and 2002 epizootics in Europe. We also investigated whether any differences in sequences between the PDV/USA2006 and the 2002 and 1988 viruses were likely to have occurred through sequencing errors, their tissue of origin, or adaption to Vero cells. Sequence information, when available for the phosphoprotein (P) membrane fusion (F), and internal matrix (M) protein genes also were compared for various viruses from outbreaks in Europe, the United States, and Canada during 1988–2006.
Cells and Tissues
Vero and VeroDogSLAM (VDS) cells were grown in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA, USA) supplemented with 5% fetal bovine serum. A blood sample from an infected seal from the 1988 epizootic was obtained from Albert Osterhaus, Erasmus University (Rotterdam, the Netherlands). Brain tissue from a harbor seal (designated PDV/3541UK) that was found off the coast of Scotland at the end of the 2002 epizootic and was PCR positive for PDV in the brain but not other tissues (lung, spleen, and lymph nodes) was obtained from Paul Jepson, Institute of Zoology, Zoological Society of London.
Reverse Transcription–PCR and DNA Sequencing
Total RNA was extracted from infected cells and tissues by using TRIzol reagent (Invitrogen). cDNA synthesis was conducted by using oligo-dT primers and the SuperScript First-Strand Synthesis kit (Invitrogen). PCR was performed by using the High Fidelity Taq kit (Invitrogen). Morbillivirus universal P gene and β-actin primers (14) and further PDV primers to the H, F, M, and P genes designed to previously published PDV sequences are given in the Table (Table). DNA sequencing was performed by using a BigDye 3.1 Terminator Cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) with primers listed in the Table. Completed PCR products were sent to the Genomics Core Facility, Queens University (Belfast, UK) for chromatographic preparation.
Isolation and Identification of USA 2006 as PDV
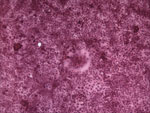
Signaling lymphocyte activation molecule (SLAM) is a receptor for both vaccine and wild-type strains of measles virus and for canine distemper and rinderpest morbilliviruses (15,16). VDS cells have been used successfully to isolate PDV from experimentally infected ferrets (Mustela putorius furo) (17), and we recently confirmed that this molecule also is used as a receptor for PDV (M. Melia et al., unpub. data). The USA 2006 virus was isolated by inoculating homogenized liver tissue from a harbor seal onto VDS cells, which resulted in syncytia formation (Figure 1). Reverse transcription–PCR (RT-PCR) was initially conducted by using previously published morbillivirus universal P gene primers (14). The PCR product was sequenced and aligned with morbillivirus sequences for this target region and showed 100% homology with PDV (data not shown).
Origin of Sequence Data
Selected gene sequences from the PDV/Ulster 88, PDV/NL88, PDV/DK 88, and PDV/DK2002 viruses from Europe were available for comparison with the PDV/USA2006 strain (10,18–21). In addition, we included partial P gene sequences obtained from tissues and nasal swabs of northern seas otters (Enhydra lutris) from an unusual mortality event in south-central Alaska in 2006, as well as samples from a harp seal found in the Gulf of St. Lawrence (Canada) in 1991 and from a hooded seal on the New Jersey, USA, coast in 1998 (22,23).
Because of improvements in techniques of sequence determination from those available in 1988, we initially resequenced the PDV/Ulster88 virus H, M, and F genes. We have designated this “new” sequence PDV/Ulster88n. For the same reason, and to determine whether adaptation to Vero cells changed the virus sequence, particularly in the cell receptor attachment H protein, we obtained RNA directly from a blood sample used to isolate the PDV/NL88 strain and conducted RT-PCR directly. We designated this “new” sequence for the H, F, and M genes as PDV/NL88n. The sequence obtained from the central nervous system tissue of the seal from the 2002 epizootic is designated PDV/3541UK.
Nucleotide and Deduced Amino Acid Sequence Comparison
Previous studies have shown that the H protein is the most variable and that comparison of the PDV/2002 and PDV88 sequences from Europe showed 8 minor amino acid changes (10,21). The sequences for the PDV/Ulster88 and PDV/NL88 viruses had been obtained from isolates in Vero (African green monkey kidney) cells, unlike the 2002 sequences, which were obtained directly from lung tissue. To determine whether errors may have occurred in the PDV/Ulster88 sequence, we initially compared the original PDV/Ulster88 and new PDV/Ulster88n H gene sequences. Changes in PDV/Ulster88 compared with the consensus sequence for all the viruses at bases 910, 911, 1134, and 1135 are not mirrored in the PDV/Ulster88n sequence, which indicates that these are likely to have resulted from sequencing errors and therefore do not reflect real differences in the more recent isolates. Similarly, to determine whether changes in the original PDV/NL88 strain are likely to have resulted from adaption to Vero cells, we compared the original PDV/NL88 H sequence with PDV/NL88n, which was amplified directly from a blood sample. Differences of PDV/NL88 from the consensus at bases 23 and 1711 are not reflected in the PDV/NL88n sequence, which indicates that these are likely to have resulted from tissue culture adaptation and/or sequencing errors.
The newly isolated PDV/USA2006 virus has 11 aa changes in the H gene (GenBank accession no. 1375698), compared with the PDV/DK2002 strain at codons 176, 200, 218, 221, 276, 327, 399, 432, 561, 561, and 576. However, the sequences at codons 200, 218, 276, 399, and 432 are in common with the PDV/NL88n strain. Similarly, silent mutations at nt 180, 564, and 1728 are the same as PDV/NL88n. With the exception of codon 564, all other differences of PDV/USA2006, compared with PDV/DK2002, reflect the consensus sequence. All of these observations suggest that the similarities of PDV/USA2006 to the 1988 virus may be due to circulation of multiple lineages. A phylogenic analysis for the H gene is shown in Figure 2, panel A.
Few amino acid changes are found in the P (GenBank accession no. 1375683) and M (Figure 3) genes of PDV/USA2006, compared with the other viruses. Complete P gene sequences were available for the DK2002, NL2002, Ulster, NL and DK 88 viruses. Partial sequences from the 1991 Canadian and 1998 USA viruses, as well as the sea otter 2006 virus, also were used for comparison (22,23). The USA/2006 P gene differs from the more recent 2002 viruses at codon 152 but is the same as all three 1988 isolates. Other minor variations between strains at the sequence level align PDV/USA2006 most closely to PDV/NL88. A phylogenic analysis based on the P gene is shown in Figure 2, panel B, and a concatenated tree based on both the H and P genes (for viruses where both sequences are available) is shown in Figure 2, panel C. The M and F gene sequence of PDV/USA2006 were compared with the sequences of PDV/Ulster88n, PDV/NL88n, PDV/DK88, and PDV/3541UK. The PDV/USA2006 M and F protein amino acid sequences (Figures 3, 4) reflect at least 1 of the other viruses at all positions. PDV/Ulster 88 M sequence differs from the other viruses at codon 84, whereas PDV/NL88n differs at codons 178, 310, 331, and 333. The first 233 nt of the PDV/3541UK M gene could not be amplified. However, alignment of the remaining sequence showed amino acids to be in common with >1 of the other viruses. In the F gene, an amino acid change occurred in the initiation codon from Met to Val in PDV/3541UK, compared with the other viruses, and silent mutations occurred at codons 20, 22, 51, 156, 198, 440, and 528.
We confirmed that at least some of the deaths in seals that occurred around Maine and Massachusetts in 2006 resulted from PDV infection and conducted sequence alignments with other strains derived during 1988–2006. Müller et al. reported 8 aa changes in the H gene between the 1988 and 2002 European strains of PDV (21). We have shown that 4 base changes in PDV/Ulster88, compared with the 2002 isolates, probably resulted from errors by resequencing this virus (PDV/Ulster88n) by using current technology. Furthermore, the PDV/NL88 strain when reisolated directly from a blood sample (PDV/NL88n) also showed fewer differences to 2002 strains, which in this case may have been due to adaptation to Vero cells, although sequencing errors cannot be ruled out. Phylogenetic analysis of the individual H and P gene sequences, as well as combined concatonated sequences (Figure 2), suggests that PDV/USA2006 is more closely related to PDV/NL88n than to the 2002 European viruses. Although sequence information was limited, we found that the 2006 sea otter virus P gene sequence was identical to that of the PDV/DK2002 virus. Therefore, the two 2006 viruses, 1 each from the US Atlantic and Pacific coasts, might have had different origins, but this possibility requires further investigation. The limited sequence information for the P gene of the 1991 virus from Canada and the 1998 virus from the United States is identical to that of the P gene of PDV/DK2002. More sequence information for these North American viruses, particularly for the H gene, might give further insight into their origin and evolution.
Unlike the 2002 viruses, from which RNA sequences were obtained directly from tissues, the PDV/USA2006 strain was isolated into VDS cells, which might explain the differences, particularly in the H gene. Sequencing viruses isolated in these cells is preferable to sequencing them isolated in Vero cells because of expression of the SLAM receptor. The SLAM binding site of the H protein has been shown to be conserved across all known morbilliviruses (24). We found that the residues were conserved in all of the PDVs tested, including the 1988 strains that had been isolated in Vero cells. Nielsen et al. (10) suggested that the higher identity of the H protein in 1988 strains than in PDV/DK2002 could have been due to passaging of the former in Vero cells. By comparing directly amplified PDV/NL88n sequences from a blood sample, we demonstrated that the similarities with the USA/2006 isolate are unlikely to have resulted from tissue culture adaption of the latter.
The M and F amino acid sequences are in common with >1 of the other viruses. The first 233 nt of the M gene of PDV/3541UK could not be amplified despite successful amplification of the F gene and the housekeeping mRNA β-actin. Therefore, mutations may remain to be identified, and those mutations may account for the lack of primer specificity in this region.
A total of 8 mutations were found in the PDV/3541UK F sequence, compared with those of PDV/USA2006, PDV/Ulster88n, and PDV/NL88n. Seven of these changes were silent. The substitution of Met to Val in the first codon may be particularly noteworthy. Perhaps, in PDV/3541UK, GUG can be used as the initiation codon or initiation may take place at the next available Met. In the latter case, a fully functional F protein would not be produced in the brain of this animal. Truncations, mutations, and deletions in the cytoplasmic domain of the F protein occur in the persistent measles central nervous system complications (subacute sclerosing panencephalitis and measles inclusion body encephalitis) (25). Sequencing of more F genes from seal brain tissue is necessary to determine whether the observed substitutions are a common feature and whether they are associated with persistent infection.
In this study we confirmed that the virus isolated from the US 2006 unusual mortality event in seals was PDV. The similarity of this isolate to the PDV/NL88n virus suggests that PDV/USA2006 may have reemerged independently of the 2002 PDVs and that multiple lineages of PDV may be circulating enzootically among the large populations of North American seals. Multiple lineages of canine distemper virus occur worldwide and affect different carnivore species (26). The Maine 2006 unusual mortality event in seals never progressed to a full-blown epizootic as occurred in Europe, perhaps because of differences in the pathogenicity of the US 2006 virus itself, or more likely, because several PDV viruses are circulating among populations of seals that are large enough to maintain them enzootically without large-scale die-offs. Contributing to the early detection of PDV in the affected seals were range extension and rapidly increasing populations of harbor and gray seals (13) that may have had a high prevalence of immunologically naive individuals, coupled with an active surveillance program to rehabilitate stranded seals. The findings of increased base substitutions in the F gene and lack of an amplifiable product at the start of the M gene from brain tissue of an animal in 2002, compared with those obtained from lung, liver, or blood, raises the possibility that mutations could occur in the central nervous system and might be associated with persistent infection.
Dr Earle is a research officer in the School of Medicine, Dentistry and Biomedical Sciences, Queen’s University Belfast, Northern Ireland, UK. His research focuses on morbilliviruses.
Acknowledgments
We thank Keith A. Matassa for supplying us with seal tissue samples from the 2006 outbreak.
This study was supported by the Marine Mammal Unusual Mortality Event Contingency Fund, USA, Department of Fisheries and Oceans Canada and the Department of Education and Learning, Northern Ireland, UK. The Department of Fisheries, Environment and Agriculture, UK, funded the collection of tissues in the seal study in Scotland. M.M.M.’s work was supported by a postgraduate studentship from the Department of Education and Learning,
References
- Osterhaus ADME, Vedder EJ. Identification of a virus causing recent distemper deaths. Nature. 1988;335:20. DOIPubMedGoogle Scholar
- Cosby SL, McQuaid S, Duffy N, Lyons C, Rima BK, Allan GM, Characterisation of a seal morbillivirus. Nature. 1988;336:115–6. DOIPubMedGoogle Scholar
- Mahy BWJ, Barrett T, Evans S, Anderson EC, Bostock CJ. Characterization of a seal morbillivirus. Nature. 1988;336:115. DOIPubMedGoogle Scholar
- Markussen NH, Have P. Phocine distemper virus infection in harp seals (Phoca groenlandica). Mar Mamm Sci. 1992;8:19–26. DOIGoogle Scholar
- Kennedy S. A review of the 1988 European seal morbillivirus epizootic. Vet Rec. 1990;127:563–7.PubMedGoogle Scholar
- Jensen T, van de Bildt M, Dietz HH, Andersen TH, Hammer AS, Kuiken T, Another phocine distemper outbreak in Europe. Science. 2002;297:209. DOIPubMedGoogle Scholar
- Barrett T, Sahoo P, Jepson PD. Seal distemper outbreak 2002. Microbiology Today. 2003;30:162–4.
- Härkönen T, Dietz R, Reijnders P, Teilmann J, Harding K, Hall A, The 1988 and 2002 phocine distemper virus epidemics in European harbor seals. Dis Aquat Organ. 2006;68:115–30. DOIPubMedGoogle Scholar
- Blixenkrone-Möller M, Svansson V, Appel M, Krogsrud J, Have P, Orvell C. Antigenic relationships between field isolates of morbilliviruses from different carnivores. Arch Virol. 1992;123:279–94. DOIPubMedGoogle Scholar
- Nielsen L, Arctander P, Jensen TH, Dietz HH, Hammer AS, Banyard AC, Genetic diversity and phylogenetic analysis of the attachment glycoprotein of phocine distemper viruses of the 2002 and 1988 epizootics. Virus Res. 2009;144:323–8. DOIPubMedGoogle Scholar
- Duignan PJ, Sadove S, Saliki JT, Geraci JR. Phocine distemper in harbor seals (Phoca vitulina) from Long Island, New York. J Wildl Dis. 1993;29:465–9.PubMedGoogle Scholar
- Duignan PJ, Saliki JT, St Aubin DJ, Early G, Sadove S, House JA, Epizootiology of morbillivirus infection in North American harbor seals (Phoca vitulina) and grey seals (Halichoerus grypus). [PMID: 8592380]. J Wildl Dis. 1995;31:491–501.PubMedGoogle Scholar
- Payne PM, Selzer LA. The distribution, abundance and selected prey of harbor seal, Phoca vitulina concolor, in southern New England. Mar Mamm Sci. 1989;5:173–92. DOIGoogle Scholar
- Galbraith SE, McQuaid S, Hamill L, Pullen L, Barrett T, Cosby SL. Rinderpest and peste de petits ruminants viruses exhibit neurovirulence in mice. J Neurovirol. 2002;8:45–52. DOIPubMedGoogle Scholar
- Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–7. DOIPubMedGoogle Scholar
- Tatsuo H, Ono N, Yanagi YJ. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J Virol. 2001;75:5842–50. DOIPubMedGoogle Scholar
- Nielsen O, Smith G, Weingartl H, Lair S, Measures L. Use of a SLAM transfected Vero cell line to isolate and characterize marine mammal morbilliviruses using an experimental ferret model. J Wildl Dis. 2008;44:600–11.PubMedGoogle Scholar
- Curran MD, O'Loan D, Rima BK, Kennedy S. Nucleotide sequence analysis of phocine distemper virus reveals its distinctness from canine distemper virus. Vet Rec. 1990;127:430–1.PubMedGoogle Scholar
- Curran MD, Lü YJ, Rima BK. The fusion protein gene of phocine distemper virus: nucleotide and deduced amino acid sequences and a comparison of morbillivirus fusion proteins. Arch Virol. 1992;126:159–69. DOIPubMedGoogle Scholar
- Blixenkrone-Möller M, Sharma B, Varsanyi TM, Hu A, Norrby E, Kövamees J. Sequence analysis of the genes encoding the nucleocapsid protein and phosphoprotein (P) of phocid distemper virus, and editing of the P gene transcript. J Gen Virol. 1992;73:885–93. DOIPubMedGoogle Scholar
- Müller G, Wohlsein P, Beineke A, Haas L, Greiser-Wilke I, Siebert U, Phocine distemper virus: characterization of the morbillivirus causing the seal epizootic in northwestern Europe in 2002. Arch Virol. 2008;153:951–6. DOIPubMedGoogle Scholar
- Goldstein T, Mazet JA, Gill VA, Doroff AM, Burek KA, Hammond JA. Phocine distemper virus in northern sea otters in the Pacific Ocean, Alaska, USA. Emerg Infect Dis. 2009;15:925–7. DOIPubMedGoogle Scholar
- Lipscomb TP, Mense MG, Habecker PL, Taubenberger JK, Schoelkopf R. Morbilliviral dermatitis in seals. Vet Pathol. 2001;38:724–6. DOIPubMedGoogle Scholar
- Hashiguchi T, Kajikawa M, Maita N, Takeda M, Kuroki K, Sasaki K, Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc Natl Acad Sci U S A. 2007;104:19535–40. Epub 2007 Nov 14. DOIPubMedGoogle Scholar
- Schmid A, Spielhofer P, Cattaneo R, Baczko K, ter Meulen V, Billeter MA. Subacute sclerosing panencephalitis is typically characterized by alterations in the fusion protein cytoplasmic domain of the persisting measles virus. Virology. 1992;188:910–5. DOIPubMedGoogle Scholar
- Martella V, Elia G, Lucente MS, Decaro N, Lorusso E, Banyai K, Genotyping canine distemper virus (CDV) by a hemi-nested multiplex PCR provides a rapid approach for investigation of CDV outbreaks. Vet Microbiol. 2007;122:32–42. DOIPubMedGoogle Scholar
Figures
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 17, Number 2—February 2011
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
S. Louise Cosby, Centre for Infection and Immunity, School of Medicine, Dentistry and Biomedical Sciences, Queen’s University Belfast Medical Biology Centre, 97 Lisburn Rd, Belfast BT9 7BL, Northern Ireland, UK
Top