Volume 17, Number 5—May 2011
Research
Experimental Oral Transmission of Atypical Scrapie to Sheep
Cite This Article
Citation for Media
Abstract
To investigate the possibility of oral transmission of atypical scrapie in sheep and determine the distribution of infectivity in the animals’ peripheral tissues, we challenged neonatal lambs orally with atypical scrapie; they were then killed at 12 or 24 months. Screening test results were negative for disease-specific prion protein in all but 2 recipients; they had positive results for examination of brain, but negative for peripheral tissues. Infectivity of brain, distal ileum, and spleen from all animals was assessed in mouse bioassays; positive results were obtained from tissues that had negative results on screening. These findings demonstrate that atypical scrapie can be transmitted orally and indicate that it has the potential for natural transmission and iatrogenic spread through animal feed. Detection of infectivity in tissues negative by current surveillance methods indicates that diagnostic sensitivity is suboptimal for atypical scrapie, and potentially infectious material may be able to pass into the human food chain.
Since the discovery of atypical scrapie (1) and its subsequent identification, mostly through active surveillance, in several countries (some with no previous history of transmissible spongiform encephalopathies [TSEs]) (2,3) such as New Zealand (4) and Australia (5), scientists have debated whether this form of TSE is in fact spontaneous or acquired (3,4,6–8) rather than contagious. The epidemiologic studies that have been undertaken suggest that atypical scrapie does not appear be transmitted between animals in the field situation (7,8). Although the routes by which natural transmission occurs have never been fully established for TSEs, it is widely accepted that ingestion of infective material, i.e., the oral route, is a key component in some TSEs, e.g., kuru (9), variant Creutzfeldt-Jakob disease (10), bovine spongiform encephalopathy (11), and transmissible mink encephalopathy (12).
Within the sheep population, susceptibility to particular strains of TSE has been shown to be heavily affected by polymorphisms of the prion protein gene of the sheep (13–18). The successful transmission of atypical scrapie to sheep after intracerebral inoculation has been previously reported for sheep of 1 genotype (A136H154Q171/A136H154Q171) (19,20), and challenges in other homologous and heterologous genotype combinations are ongoing. However, successful intracerebral transmission of a particular TSE agent in a particular species does not necessarily indicate susceptibility by the oral route (21).
The tissue distribution of infectivity or disease-specific prion protein (PrPSc) in bovine spongiform encephalopathy in sheep has led to extensive public health control measures based on the known pathogenesis and distribution of PrPSc in edible tissues, and their removal from carcasses of animals over a certain age. Classic scrapie may also show the widespread accumulation of PrPSc in peripheral tissues. Although early studies of atypical scrapie did not show PrPSc or infectivity outside the brain, recent data indicate that peripheral tissues from naturally infected animals can harbor infectivity either in the presence or absence of PrPSc (22). However, whether this infectivity is established before or after the agent has propagated in the central nervous system is unknown.
The first aim of the current study was to examine the distribution of infectivity in peripheral tissues in animals at and beyond the cutoff point for the current meat hygiene regulations of the European Commission (i.e., 12 months of age). The second aim was to investigate the potential for oral transmission of atypical scrapie.
Recipient Animals
Six Cheviot lambs with PrP genotype AHQ/AHQ and 6 Poll Dorset lambs with the genotype ARR/ARR were sourced from the New Zealand–derived flock owned by the UK Department of Food, Environment and Rural Affairs (3), and transported in utero with their dams to the Veterinary Laboratories Agency–Weybridge before lambing. All procedures involving animals were carried out in accordance with the UK Animal (Scientific Procedures) Act 1986, under license from the UK Government Home Office.
Experimental Challenge
Within 24 hours of birth, during February–March 2008, each lamb was challenged orally with 2.5 g of brain homogenate prepared from animals with naturally acquired cases of atypical scrapie of the same genotypes, and again with a further 2.5 g of the same homogenate 14 days later. Homogenate was delivered through a syringe to the oropharynx (23). The lambs were kept with their dams and separate from each other until they were weaned at 9–11 weeks of age; after weaning, they were housed in groups according to genotype.
Donor Animals
Inoculum ARRa
This animal was identified through passive surveillance in 2004. The sample was submitted before ELISA screening was used routinely for passive surveillance, and no inoculum remained for further biochemical characterization after the challenge of 5 lambs. Results of immunolabeling in the medulla, cerebellum, and thalamus, and Western blot on the medulla were consistent with atypical scrapie (24) (data not shown).
Inoculum ARRb
This animal was identified through active surveillance (fallen stock). ELISA (Bio-Rad Laboratories, Marnes-la-Coquette, France) of this inoculum gave optical density (OD) values of 1.554 and 0.761. The OD of the initial diagnostic screening sample was 1.074. Results of Western blot and immunolabeling throughout the neuraxis were consistent with atypical scrapie (data not shown).
Inoculum AHQ
This animal was identified through active surveillance (fallen stock). ELISA (Bio-Rad Laboratories) of inoculum prepared from this animal gave a positive OD reading of 2.272. The initial diagnostic screening sample gave an ELISA OD of 0.948. Western blot and immunolabelling results were consistent with atypical scrapie, but with a preponderance of white matter staining and substantially less neuropil staining than has been seen in other AHQ atypical cases (data not shown).
Clinical Monitoring
All animals were monitored daily during routine husbandry procedures and monthly during blood sampling from 8 months post inoculation. Within 12 days of the cull date for each sheep, a clinical and neurologic examination was carried out, including cranial nerve assessment and testing of response to scratching of the back according to published protocols (23). On the basis of clinical signs, the animal’s clinical TSE status was categorized as follows: normal (no apparent signs of scrapie), inconclusive with regards to scrapie (e.g., impaired menace response, minor wool loss), and suspected scrapie (combination of abnormalities in behavior, sensation, or movement), as has been described for goats (25).
Cull Schedule and Sampling
Three animals of each genotype were selected at random and culled at 12 months of age, and the remaining 3 were culled at 24 months of age. Animals were euthanized by using quinalbarbitone sodium (Somulose–Dechra Veterinary Products, Shrewsbury, UK), and a variety of samples of neural and non-neural tissues (Table A1) were obtained by using aseptic techniques and either placed into 10% formal saline (neural tissues), 10% buffered formalin (non-neural tissues) or frozen and held at –80°C for subsequent examination by immunohistochemical (IHC) testing, ELISA, or both (Table), depending on the size and nature of the sample. Some samples were collected for examination in the event of a positive result in the samples chosen for initial screening.
Immunohistochemical Testing
Fixed samples were processed into paraffin wax, sectioned, and stained with hematoxylin and eosin as described (26). IHC labeling to detect PrPSc was performed as previously described (19) by using mouse monoclonal antibody 2G11 (Institut Pourquier, Montpellier, France), raised against ovine PrP peptide sequence 146-R154 R171–182. IHC profiles were created by using a standard subjective method as previously described (24) in which the type of PrPSc immunolabeling is assessed in a standard range of precise neuro-anatomical areas. Tissues from the lympho-reticular system of each challenged animal were examined by IHC as described above.
ELISA
All tissues were analyzed by using the TeSeE kit (Bio-Rad Laboratories) for TSE detection in sheep and goats, according to the manufacturer’s instructions. The cutoff value was calculated as the average absorbance reading of the negative control values plus a value of 0.14 absorbance units. To directly measure PrPSc content in the samples that were used to inoculate the recipient animals, the inocula were prepared for the Bio-Rad protocol before analysis to account for variation in tissue and buffer content. Samples were centrifuged at high speed to concentrate insoluble material. The weight of the pellet was calculated, and then it was resuspended in homogenization buffer to prepare a 20% wt/vol homogenate and continued as described from the ribolysation stage. Pellets that contained insufficient weight to prepare 250 μL of 20% wt/vol homogenate were supplemented by using brain homogenate or relevant tissue prepared from sheep that had not been exposed to scrapie (control reference material) before testing.
Western Blot
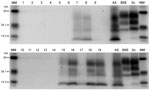
Positive, fresh brain samples were subjected to the TeSeE Universal Western blot (Bio-Rad Laboratories, catalog no. 355 1169) as described (19). Molecular mass markers were included at either end of the gel. A single lane each of samples from animals with known UK classic scrapie, known UK bovine BSE, and a known UK atypical scrapie were included for profile comparisons.
Mouse Bioassay
Brain areas from animals positive for PrPSc were assayed in mice to assess the stability of agent following passage. In addition, bioassay of 3 additional tissues (cerebellum, spleen, and distal ileum) has also been initiated from every challenged sheep whether all tests were negative or not (Table A1). Samples from the donor and experimental recipient sheep were treated in the same way. Tissue homogenate (10%) was prepared wt/vol in normal saline, screened for microbiologic sterility by using standard methods, treated using ampicillin and gentamicin if contamination was identified, and rechecked before use.
Panels of 10 transgenic mice overexpressing ovine prion protein gene (Tg338 [27]) were inoculated intracerebrally with 20 μL and, when sufficient inoculum was available, intraperitoneally with 100 μL of homogenate. Mice were monitored weekly and were killed when they had shown clinical signs on 2 of 3 consecutive monitoring days or had reached their natural lifespan. Brains were then fixed and processed, and lesion profiles were produced as described in detail elsewhere (28).
Full details of genotype, clinical status, kill time points, and test results for each animal are shown in the online Table A1. A proportion of the mouse infectivity bioassays are incomplete, as indicated in the text below, so some tissues that are currently considered negative for PrPSc by bioassay may change status at a later date.
Cull at 12 Months
Of the 6 animals (3 ARR/ARR and 3 AHQ/AHQ) killed after 12 months, 5 were clinically healthy at the time of culling. Animal 3 appeared nervous during handling, although its behavior at previous blood sampling sessions had been unremarkable, and it displayed a bilaterally absent menace response (inconclusive results with regards to scrapie).
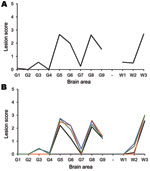
None of the sheep exhibited PrPSc in the tissues examined. Bioassays in mice of the cerebellum, spleen, and distal ileum from each animal are ongoing. To date, infectivity has been demonstrated in the cerebellum of animal 1 and the distal ileum of animals 8 and 9. Only the bioassay from animal 9 has been completed, with 9 mice succumbing to disease (Figure 1). A proportion of the mice used in the assays of samples from animals 1 and 8are still alive.
Cull at 24 Months
[[AA:FA:PREVIEWHTML]]Of the 6 sheep culled at 24 months post inoculation, 3 appeared clinically normal. Animal 6 displayed alopecia suggestive of pruritus although no pruritic behavior was observed (inconclusive with regards to scrapie). Animal 10 appeared nervous when it was approached and handled, which had not been observed on previous blood sampling sessions, and displayed a wide-based hind limb posture (inconclusive with regards to scrapie). Animal 12 appeared nervous with a fine head tremor during handling, had a wide-based hind limb posture, and was ataxic with uncoordinated jumps, swaying, and loss of balance (Appendix Video). At blood sampling 2 months earlier, the sheep had been observed circling clockwise when left alone, which was not seen at the final examination (suspected scrapie). None of the sheep had a positive scratch test, and none of these 6 sheep had lost weight before cull.
PrPSc was detected in 2 animals only, both of which were AHQ homozygotes. In animal 11, which was clinically normal, PrPSc was observed in the caudal thalamus, restricted to the lamina medullaris externa, in the caudate nucleus, the amygdala, and external capsule, and minimal labeling was found in the basal and septal nuclei. Positive immunolabeling (not shown) was seen in the hippocampus, together with extensive incidental “thready” staining (29). White matter labeling was found in the olfactory tract and rostral commissure (Figure 2). No PrPSc was detected in the medulla or cerebellum, the areas currently used for statutory surveillance purposes. In animal 12, PrPSc was distributed widely throughout the brain and was detectable by IHC (Figure 2), ELISA, and Western blot (Figure 3). Widespread white matter labeling and mild-to-moderate granular labeling were found, consistent with that described for natural cases of atypical scrapie (24) (Figure 2). No evidence of PrPSc accumulation was found in any of the examined tissues from the other animals. Western blot profiles of PrPSc from these animals were compatible with those of animals with of atypical scrapie (Figure 3).
In addition to the tissues listed in the Table, the jejunum, lateral retropharyngeal lymph node, respiratory epithelium, triceps muscle, cranial cervical ganglion, nodose ganglion, facial nerve, trigeminal ganglion, and sciatic nerve have all been screened by IHC in the PrPSc-positive animals. No evidence of PrPSc accumulation outside the brain has been identified in these animals.
Bioassays of cerebellum, spleen, distal ileum, and of tissues with detectable PrPSc are still ongoing. To date, the cerebellum sample from animal 11 and the distal ileum samples from animal 12 have resulted in PrPSc-positive mice, although no PrPSc was detected in those tissues (Table A1). When assays are complete, the lesion profiles obtained in the mice show that the biological profile of the experimental animals is the same as that of the donor, and that infectivity detected in the peripheral tissues has the same biologic signature as that in the brain (Figure 1).
This study is still ongoing and will not be completed until 2012. However, the current interim report documents the successful oral transmission of atypical scrapie, confirms that the disease phenotype is retained following transmission by this route in AHQ/AHQ sheep, and indicates that infectivity can be demonstrated in the gut in the absence of detectable PrPSc at least as early as 12 months after exposure.
One sheep (animal 12) culled at 24 months post inoculation displayed abnormalities in behavior and movement suggestive of atypical scrapie. Signs like ataxia with head tremor and circling have been described in experimental (19) and natural (3,30) disease, which was attributed to lesions in the cerebellum and forebrain, respectively, corresponding with PrPSc accumulation in these areas (20,24).
By contrast, animal 11, which had confirmed atypical scrapie based on postmortem tests, was considered clinically normal. The less severe and limited PrPSc accumulation in the brain of this sheep than in animal 12 may explain the absence of clinical abnormalities, which is supported by our findings in goats with scrapie in which more extensive PrPSc accumulation in the brain was usually associated with a more severe clinical disease (25).
Although all TSEs are transmissible after intracerebral challenge to a susceptible host, only some are infectious under natural conditions. Therefore, it was important from a pathogenesis and disease control perspective to establish whether or not oral transmission can be successful. However, the challenge model in this study exposed animals as neonates, when the esophageal groove is operational and the lambs are physiologically monogastric. Exposure of 3-month-old ruminating animals to similar amounts of positive brain by the oral route have so far not resulted in any clinical disease, with all animals still alive >1,500 days post challenge (M.M. Simmons, unpub. data), but most natural cases have been recorded in animals older than this, so these animals may still progress to disease in the next few years. Since this challenge study in older animals has no time-kill component, and no losses caused by unrelated disease have occurred, whether any of these sheep are in a preclinical phase of disease is unknown. Unfortunately, the absence of detectable PrPSc in lymphoreticular tissues of sheep with atypical scrapie precludes the use of biopsies to ascertain early infection in these animals.
Transmission may be more efficient in newborn animals; the incubation periods of sheep orally infected with classical scrapie were significantly shorter in sheep challenged at 14 days of age than those challenged at 6 months of age (31). If, however, oral transmission is only effective in such young animals, then field exposure would most likely have to be through milk, which is known to be a highly effective route of transmission for classical scrapie (32). No data are currently available on the potential infectivity of milk from animals with atypical scrapie.
Successful oral transmission also raises questions regarding the pathogenesis of this form of disease. There must be passage of the infectious agent from the alimentary canal to the brain through one of several possible routes, most likely those that have been suggested and discussed in detail for other TSEs, for example, retrograde neuronal transportation either directly (33–35) or through lymphoid structures or hematogenously (36). Infectivity in the absence of readily demonstrable PrPSc has been reported (37–39), and although the mouse bioassay may detect evidence of disease in other tissues, these data may not be available for at least another 2 years. More protease-sensitive forms of PrPSc may be broken down more efficiently within cells and thus do not accumulate in peripheral tissues (19), enabling atypical PrPSc to transit the digestive tract and disseminate through other systems in small amounts before accumulating detectably in the central nervous system.
Although we do not have epidemiologic evidence that supports the efficient spread of disease in the field, these data imply that disease is potentially transmissible under field situations and that spread through animal feed may be possible if the current feed restrictions were to be relaxed. Additionally, almost no data are available on the potential for atypical scrapie to transmit to other food animal species, certainly by the oral route. However, work with transgenic mice has demonstrated the potential susceptibility of pigs, with the disturbing finding that the biochemical properties of the resulting PrPSc have changed on transmission (40). The implications of this observation for subsequent transmission and host target range are currently unknown.
How reassuring is this absence of detectable PrPSc from a public health perspective? The bioassays performed in this study are not titrations, so the infectious load of the positive gut tissues cannot be quantified, although infectivity has been shown unequivocally. No experimental data are currently available on the zoonotic potential of atypical scrapie, either through experimental challenge of humanized mice or any meaningful epidemiologic correlation with human forms of TSE. However, the detection of infectivity in the distal ileum of animals as young as 12 months, in which all the tissues tested were negative for PrPSc by the currently available screening and confirmatory diagnostic tests, indicates that the diagnostic sensitivity of current surveillance methods is suboptimal for detecting atypical scrapie and that potentially infectious material may be able to pass into the human food chain undetected.
Acknowledgments
We thank the staff of the Agricultural Development and Advisory Service, Arthur Rickwood Sheep Unit, and the Animal Services Unit at the Veterinary Laboratories Agency for their provision and dedicated care of the animals, and the staff of the Neuropathology and Histopathology sections of the Pathology Department, and the Molecular Pathogenesis Department, in particular Thomas Holder, for excellent scientific support.
This work was supported by the Food Standards Agency (Project MO 3057).
References
- Benestad SL, Arsac JN, Goldmann W, Nöremark M. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet Res. 2008;39:19. DOIPubMedGoogle Scholar
- Epstein V, Pointing S, Halfacre S. Atypical scrapie in the Falkland Islands. Vet Rec. 2005;157:667–8.PubMedGoogle Scholar
- Simmons HA, Simmons MM, Spencer YI, Chaplin MJ, Povey G, Davis A, Atypical scrapie in sheep from a UK research flock which is free from classical scrapie. BMC Vet Res. 2009;5:8. DOIPubMedGoogle Scholar
- Kittelberger R, Chaplin MJ, Simmons MM, Ramirez-Villaescusa A, McIntyre L, MacDiarmid SC, Atypical scrapie/Nor98 in a sheep from New Zealand. J Vet Diagn Invest. 2010;22:863–75. DOIPubMedGoogle Scholar
- The Australian. Scrapie, atypical, ovine—Australia: (Western Australia) suspected. ProMed. 2010 Mar 12. http://promedmail.org, archive no. 20100312.0803.
- Baron T, Biacabe AG, Arsac JN, Benestad S, Groschup MH. Atypical transmissible spongiform encephalopathies (TSEs) in ruminants. Vaccine. 2007;25:5625–30. DOIPubMedGoogle Scholar
- Benestad SL, Arsac JN, Goldmann W, Nöremark M. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet Res. 2008;39:19. DOIPubMedGoogle Scholar
- Fediaevsky A, Gasqui P, Calavas D, Ducrot C. Discrepant epidemiological patterns between classical and atypical scrapie in sheep flocks under French TSE control measures. Vet J. 2010;185:338–40. DOIPubMedGoogle Scholar
- Lindenbaum S. Cannibalism, kuru and anthropology. Folia Neuropathol. 2009;47:138–44.PubMedGoogle Scholar
- Ghani AC, Donnelly CA, Ferguson NM, Anderson RM. The transmission dynamics of BSE and vCJD. C R Biol. 2002;325:37–47. DOIPubMedGoogle Scholar
- Wilesmith JW, Ryan JB, Atkinson MJ. Bovine spongiform encephalopathy: epidemiological studies on the origin. Vet Rec. 1991;128:199–203. DOIPubMedGoogle Scholar
- Burger D, Hartsough GR. Encephalopathy of mink. II. Experimental and natural transmission. J Infect Dis. 1965;115:393–9. DOIPubMedGoogle Scholar
- Hoinville LJ. A review of the epidemiology of scrapie in sheep. Rev Sci Tech. 1996;15:827–52.PubMedGoogle Scholar
- Dawson M, Moore RC, Bishop SC. Progress and limits of PrP gene selection policy. Vet Res. 2008;39:25. DOIPubMedGoogle Scholar
- Moum T, Olsaker I, Hopp P, Moldal T, Valheim M, Moum T, Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor98 cases. J Gen Virol. 2005;86:231–5. DOIPubMedGoogle Scholar
- Saunders GC, Cawthraw S, Mountjoy SJ, Hope J, Windl O. PrP genotypes of atypical scrapie cases in Great Britain. J Gen Virol. 2006;87:3141–9. DOIPubMedGoogle Scholar
- Saunders GC, Lantier I, Cawthraw S, Berthon P, Moore SJ, Windl O, Protective effect of the T112 PrP variant in sheep challenged with BSE. J Gen Virol. 2009;90:2569–74. DOIPubMedGoogle Scholar
- Goldmann W. PrP genetics in ruminant transmissible spongiform encephalopathies. Vet Res. 2008;39:30. DOIPubMedGoogle Scholar
- Simmons MM, Konold T, Simmons HA, Spencer YI, Lockey R, Spiropoulos J, Experimental transmission of atypical scrapie to sheep. BMC Vet Res. 2007;3:20. DOIPubMedGoogle Scholar
- Simmons MM, Konold T, Thurston L, Bellworthy SJ, Chaplin MC, Moore SJ. The natural atypical scrapie phenotype is preserved on experimental transmission and sub-passage in PRNP homologous sheep. BMC Vet Res. 2010;6:14. DOIPubMedGoogle Scholar
- Matthews D, Cooke BC. The potential for transmissible spongiform encephalopathies in non-ruminant livestock and fish. Rev Sci Tech. 2003;22:283–96.PubMedGoogle Scholar
- Andréoletti O, Orge L, Benestad SL, Beringue V, Litaise C, Simon S, Atypical/Nor98 scrapie infectivity in sheep peripheral tissues. PLoS Pathog. 2011;7:e1001285. DOIPubMedGoogle Scholar
- Wells GAH, Hawkins SAC. Animal models of transmissible spongiform encephalopathies: experimental infection, observation and tissue collection. In: Lehmann S, Grassi G, editors. Methods and tools in biosciences and medicine. Techniques in prion research. Basel: Birkhäuser Verlag; 2004. p. 38–71.
- Moore SJ, Simmons M, Chaplin M, Spiropoulos J. Neuroanatomical distribution of abnormal prion protein in naturally occurring atypical scrapie cases in Great Britain. Acta Neuropathol. 2008;116:547–59. DOIPubMedGoogle Scholar
- Konold T, Bone GE, Phelan LJ, Simmons MM, González L, Sisó S, Monitoring of clinical signs in goats with transmissible spongiform encephalopathies. BMC Vet Res. 2010;6:13. DOIPubMedGoogle Scholar
- Ligios C, Jeffrey M, Ryder SJ, Bellworthy SJ, Simmons MM. Distinction of scrapie phenotypes in sheep by lesion profiling. J Comp Pathol. 2002;127:45–57. DOIPubMedGoogle Scholar
- Laude H, Vilette D, le Dur A, Archer F, Soulier S, Besnard N, New in vivo and ex vivo models for the experimental study of sheep scrapie: development and perspectives. C R Biol. 2002;325:49–57. DOIPubMedGoogle Scholar
- Beck KE, Chaplin M, Stack M, Sallis RE, Simonini S, Lockey R, Lesion profiling at primary isolation in RIII mice is insufficient in distinguishing BSE from classical Scrapie. Brain Pathol. 2010;20:313–22. DOIPubMedGoogle Scholar
- Ryder SJ, Spencer YI, Bellerby PJ, March SA. Immunohistochemical detection of PrP in the medulla oblongata of sheep: the spectrum of staining in normal and scrapie-affected sheep. Vet Rec. 2001;148:7–13. DOIPubMedGoogle Scholar
- Konold T, Davis A, Bone G, Bracegirdle J, Everitt S, Chaplin M, Clinical findings in two cases of atypical scrapie in sheep: a case report. BMC Vet Res. 2007;3:2. DOIPubMedGoogle Scholar
- Ryder SJ, Dexter GE, Heasman L, Warner R, Moore SJ. Accumulation and dissemination of prion protein in experimental sheep scrapie in the natural host. BMC Vet Res. 2009;5:9. DOIPubMedGoogle Scholar
- Konold T, Moore SJ, Bellworthy SJ, Simmons HA. Evidence of scrapie transmission via milk. BMC Vet Res. 2008;4:14. DOIPubMedGoogle Scholar
- Arnold ME, Ryan JBM, Konold T, Simmons MM, Spencer YI, Wear A, Estimating the temporal relationship between PrPSc detection and incubation period in experimental bovine spongiform encephalopathy (BSE) of cattle. J Gen Virol. 2007;88:3198–208. DOIPubMedGoogle Scholar
- Masujin K, Matthews D, Wells GA, Mohri S, Yokoyama T. Prions in the peripheral nerves of bovine spongiform encephalopathy–affected cattle. J Gen Virol. 2007;88:1850–8. DOIPubMedGoogle Scholar
- Hoffmann C, Ziegler U, Buschmann A, Weber A, Kupfer L, Oelschlegel A, Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. J Gen Virol. 2007;88:1048–55. DOIPubMedGoogle Scholar
- Sisó S, González L, Jeffrey M. Neuroinvasion in prion diseases: the roles of ascending neural infection and blood dissemination. Interdiscip Perspect Infect Dis. 2010;:747892.PubMedGoogle Scholar
- Barron RM, Campbell SL, King D, Bellon A, Chapman KE, Williamson RA, High titres of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrPSc in vivo. J Biol Chem. 2007;282:35878–86. DOIPubMedGoogle Scholar
- Wells GA, Spiropoulos J, Hawkins SA, Ryder SJ. Pathogenesis of experimental bovine spongiform encephalopathy: preclinical infectivity in tonsil and observations on the distribution of lingual tonsil in slaughtered cattle. Vet Rec. 2005;156:401–7.PubMedGoogle Scholar
- Houston F, McCutcheon S, Goldmann W, Chong A, Foster J, Sisó S, Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood. 2008;112:4739–45. DOIPubMedGoogle Scholar
- Espinosa JC, Herva ME, Andreoletti O, Padilla D, Lacroux C, Cassard H, Transgenic mice expressing porcine prion protein resistant to classical scrapie but susceptible to sheep bovine spongiform encephalopathy and atypical scrapie. Emerg Infect Dis. 2009;15:1214–21. DOIPubMedGoogle Scholar
Figures
Table
Cite This Article1Current affiliation: Murdoch University School of Veterinary and Biomedical Sciences, Murdoch, Western Australia, Australia.
Table of Contents – Volume 17, Number 5—May 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Marion M. Simmons, Department of Pathology and Host Susceptibility, VLA-Weybridge, Woodham Lane, New Haw, Addlestone, Surrey, KT15 3NB, UK
Top