Volume 18, Number 11—November 2012
Research
Livestock Density as Risk Factor for Livestock-associated Methicillin-Resistant Staphylococcus aureus, the Netherlands
Abstract
To determine whether persons living in areas of high animal density are at increased risk for carrying livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA), we used an existing dataset of persons in the Netherlands with LA-MRSA carriage and controls who carried other types of MRSA. Results of running univariate and multivariate logistic regression models indicated that living in livestock-dense areas increases the odds of nasal carriage of LA-MRSA. We found that doubling pig, cattle, and veal calf densities per municipality increased the odds of LA-MRSA carriage over carriage of other types of MRSA by 24.7% (95% CI 0.9%–54.2%), 76.9% (95% CI 11.3%–81.3%), and 24.1% (95% CI 5.5%–45.9%), respectively, after adjusting for direct animal contact, living in a rural area, and the probable source of MRSA carriage. Controlling the spread of LA-MRSA thus requires giving attention to community members in animal-dense regions who are unaffiliated with livestock farming.
Staphylococcus aureus is a zoonotic and human pathogen that can cause a range of health outcomes in humans from minor to life-threatening infections of the skin, bloodstream, respiratory system, urinary tract, and surgical sites (1). An increasing proportion of S. aureus infections involve drug-resistant strains, including methicillin-resistant S. aureus (MRSA) (2). In 2007, 171,200 MRSA infections occurred in European Union member states plus Iceland and Norway, resulting in 1,050,000 extra days spent in the hospital (3) This translates into excessive hospital inpatient and outpatient costs because of the need to isolate patients and because patients require longer stays and more extensive treatments (3). In 2005 in the United States, an estimated 94,000 MRSA infections resulted in >18,000 deaths (4).
MRSA has, in the past, been largely associated with hospitals and other healthcare facilities, but since 2000, the majority of MRSA infections in most countries are acquired in the community outside of healthcare settings (5,6). These strains of community-acquired MRSA are vital public health concerns, but less is known of their origins and routes of transmission. Among these strains of community-acquired MRSA, livestock-associated (LA) strains have been detected in several regions of the world (7).
Originally, the LA-MRSA strain studied here was denoted as nontypeable MRSA because of the inability to type it by using standard methods of pulsed-field gel electrophoresis (8). It was first detected in the Netherlands in 2003 (9) and, as of 2010, accounts for >40% of the MRSA cases in that country (10). LA-MRSA has now been identified largely as a single clonal complex on the basis of multilocus sequence typing (ST398), and this clonal complex has a demonstrated association with pigs, cows, and other animals (11,12), although other clonal complexes have also been shown to be associated with livestock as well. In several European countries, increased risks of carriage have been reported in persons in contact with pigs and veal calves, including farmers, veterinarians, and slaughterhouse workers (13,14). In the Netherlands, among these occupational groups, the prevalence of ST398 carriage is roughly 42% (15), whereas the prevalence of any strain of MRSA in the general population is <1% (16).
The emergence and transmission of LA-MRSA among humans and animals (such as poultry, horses, companion animals, pigs, and cattle) have recently been reviewed (17). Most epidemiologic studies have focuses on identifying individual and farm level characteristics associated with LA-MRSA carriage and on studying those in direct contact with livestock. In 1 study among pig farmers and their household members, 30% were carriers of MRSA ST398, and the risk for carriage was related to direct exposure to pigs (15). A study among veterinarian field workers found that after short-term occupational exposure to pigs, 17% of them carried MRSA. However, >90% of those lost this carriage the next day (18). Another study found a clear association between carriage and the duration of contact with veal calves and that carriage was strongly reduced after a period of absence from animal contact (11,19). These studies, which indicate a high risk for carriage from livestock contact but little persistence of carriage after interruption of animal contact, bring into question the ability of LA-MRSA to spread into the population. Only 1 study examined the role of living in a livestock-dense region as a risk factor as well but did not find it to be a risk factor (20). This study used a random mailing in the 3 most pig-dense municipalities in the Netherlands. Of the 534 adult respondents without livestock contact, 1 person was positive for MRSA (0.2%), compared with 13 of 49 persons who worked or lived on a livestock farm (26.5%).
In 2007, risk factors for LA-MRSA carriage in the Netherlands were investigated by van Loo and colleagues (8). They found that risk factors for increased odds of LA-MRSA carriage included contact with livestock, acquiring MRSA through known risk factors such as travel to a foreign country or recent interaction with the healthcare environment, and living in a rural area. Our study, conducted during 2008–2011, built upon this work to test the hypothesis that persons living in areas of high pig density may be at increased risk for carrying LA-MRSA. We did this by combining information about where persons lived and what the livestock density was in these areas for which existing information on risk factors had been determined in the 2007 study.
Data
We used data from van Loo et al. (8), which consisted of records of all index case-patients with nontypeable MRSA carriage (now referred to as LA-MRSA) from the Netherlands from its emergence in 2003 through September 2005, before the country adopted active surveillance of high risk populations. Information on case-patients and controls was obtained from a national MRSA surveillance program through the country’s Institute for Public Health and the Environment (www.rivm.nl/mrsa). Case-patients were LA-MRSA index patients, that is, the first in a cluster of persons who tested positive for LA-MRSA from a given reference laboratory. Each LA-MRSA case-patient was matched with 2 controls from the same laboratory. The controls were also index patients, but had tested positive for a typeable strain of MRSA (T-MRSA) instead of to LA-MRSA. Further details on subject selection can be found in the original article (8).
The same variables used in the 2007 study were used in this current study: contact with pigs, contact with cows, the probable source of MRSA, and whether one lived in a rural area. Probable source of MRSA was placed by the original authors into the following categories: healthcare setting, foreign source (such as travel to another country), other source, or an unknown source (8). Our study added municipality level variables of livestock and human population densities and location of residence of study participants. (Municipalities are administrative boundaries in the Netherlands that comprise provinces. In 2005, there were 498 municipalities.) To accomplish this, our inclusion criteria were residence in the Netherlands, availability of information on individual contact with livestock, and geographic information sufficient to support mapping each person to the 6 digit postal code of his or her residence.
We then assigned spatial coordinates to LA-MRSA case-patients and T-MRSA controls on the basis of their 6-digit postal code using the geographic information system software, ArcGIS version 9.3 (21). When this method was not sufficient, we used Google Earth (22).
We downloaded municipality level statistics of population; land area; and numbers of swine, veal calves, and cows in 2005 from CBS StatLine (23). Livestock densities and population density were calculated as the number of animals (pigs, cows, and veal calves) per hectare of land in a municipality. In ArcGIS, we determined in which municipality participants lived, and assigned to them municipality-level characteristics of animal and population densities. We determined counts of case-patients and controls for each of the municipalities using ArcGIS. This study was reviewed and approved by the Institutional Review Board at Johns Hopkins Bloomberg School of Public Health.
Statistical Analysis
Summary statistics for all relevant variables were reported by using STATA version 10 (24). We explored the spatial variation in risk for LA-MRSA—or the concept that the risk or odds of acquiring LA-MRSA varies geographically—using spatial methods available in the R statistical package (25). We estimated spatial intensity of case-patients and controls for locations across the study area, defined respectively as the expected numbers of case-patients and controls per km2. Spatially varying intensity provides an estimate of regions of high and low densities of case-patients and controls. Spatial intensity is often measured as weighted counts as described (26). We used the quartic kernel as our weighting scheme in this study. Using the intensity estimates, we calculated the spatial odds of LA-MRSA to compare the geographic variation of case-patients and controls across the study area. The spatial odds of LA-MRSA per km2 compared with those for T-MRSA per km2 were calculated as the ratio of estimated case-to-control intensities (26).
Contact with pigs, contact with cows, and rural (versus urban) residence were modeled as binary variables. Probable MRSA source was a categorical variable. We compared probable acquisition of MRSA from a foreign country, acquisition from another source, or acquisition from an unknown source with the referent group of healthcare-related acquisition.
We determined goodness of fit of the models using Akaike information criteria and the Hosmer-Lemeshow goodness-of-fit test. Likelihood ratio tests were used to compare multivariate nested models. The densities of livestock were right skewed; thus, we log-transformed the variables to create a more linear relationship between animal density and log odds of LA-MRSA. For ease of interpretation, instead of the 1 log increase in livestock densities, we used a doubling of livestock densities, which is calculated by raising 2 to the power of the β of the density coefficient in the logit model (27). Variograms were used to diagnose possible spatial variation in regression residuals, with inference on regression parameters adjusted accordingly.
In a separate but related analysis, we identified specific clusters of LA-MRSA. We used SaTScan version 9.0 (28) to conduct this cluster detection analysis with a Poisson model of counts of case-patients per municipality after adjusting for population size as described (28). SaTScan is a software package that is used to analyze spatial and temporal patterns in data. It uses a moving window method (in this application, over a set of contiguous municipalities) and determines the presence of a cluster on the basis of whether the estimated risk within a window is significantly greater than the estimated risk outside of the window. Statistical significance is based on the null hypothesis of Poisson constant risk (28). We created maps showing the identified clusters after adjustment of population density per municipality in ArcGIS. We made similar maps after further adjusting for pig, cow, and veal calf densities per municipality.
Study Population Characteristics
Descriptive statistics of the study population are shown in Table 1. From the total population used in the Van Loo analysis of 111 persons (35 case-patients, 76 controls) (8), 87 persons (27 case-patients, 60 controls) were included in our study after we excluded persons who lived outside of the Netherlands (n = 4), persons for whom spatial information was insufficient (n = 3), and persons for whom information about individual contact with livestock was lacking (n = 17).
Of the 87 subjects with complete case information, most of those who had contact with pigs (10/13, 76.9%) and cows (7/8, r 87.5%) were LA-MRSA case-patients. Three subjects had contact with both pigs and cows, 2 of whom were case-patients. Twelve of 27 persons without any direct contact with livestock were LA-MRSA positive (44.4%).
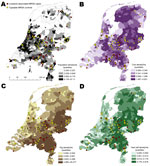
Specific locations of case-patients and controls are plotted against municipality level population (Figure 1, panel A), cow density (Figure 1, panel B), pig density (Figure 1, panel C) and veal calf density (Figure 1, panel D). Case-patients and controls had significant differences in human and livestock densities per municipality (Table 1).
Spatial Odds
Relatively high concentrations of controls are seen in general areas of high population density while higher spatial concentrations of case-patients are seen in the more agricultural areas of the country (Figure 2, panels A, B). We estimated spatial odds to give a visual assessment of the spatial variation in risk across the Netherlands (Figure 2, panel C). It is evident that the greatest differences in odds between case-patients and controls are in general areas of high pig density, as was originally reported by van Loo and colleagues (8). The elevated spatial odds in the northern part of the country are a spurious result because of small numbers of case-patients and controls and not something that we put forth as a valid result.
Univariate Logistic Regression
Univariate models results are reported in Table 2. Persons who have contact with pigs have 11.18 times the odds of carrying LA-MRSA (compared with odds of carrying T-MRSA) than persons without pig contact (95% CI 2.76–45.30; p<0.001). A similar relationship is seen when persons with and without contact with cows are compared (odds ratio [OR] 20.65; 95% CI 2.39–178.31; p <0.006). Living in a rural area rather than living in an urban area is associated with 11.2 times the odds for LA-MRSA compared with T-MRSA (95% CI 3.15–39.76; p<0.000). Carriage of MRSA from an unknown or “other” source, as compared to healthcare settings (a priori known to be associated with typeable MRSA), was significantly (p<0.05) associated with the odds of LA-MRSA carriage as compared to T-MRSA.
We found that when pig density per hectare is doubled within a municipality, the odds of acquiring LA-MRSA in a univariate model are increased by 29.5% over the odds of acquiring T-MRSA (p<0.003). Similarly, doubling cow and veal calf densities increases the odds of acquiring LA-MRSA compared with those for acquiring T-MRSA by 75.4% (p<0.001) and 19.8% (p<0.001), respectively.
When the inclusion criteria required by our study were used, 16% of original data were lost. We conducted sensitivity analyses by coding all persons with missing livestock contact information as either all having contact or as all not having contact to produce what might be bounds for high and low extremes. In all cases, livestock densities remained significant independent risk factors at the 0.05% level (Table 3).
Multivariate Models
Multivariate model results are reported in Table 4. Model 1 is based only on the original individual level variables from van Loo’s study (8): contact with pigs and cows, rural versus urban residence, and information on patient’s probable source of MRSA. In model 1, both contact with pigs and rural residence remain significant predictors as they were in the univariate models, even when adjusting for contact with cows and probable MRSA source. Odds for LA-MRSA compared with those for T-MRSA were 11.6 times higher for a foreign source of MRSA than they were with a healthcare source (95%CI 1.04–129.63; p<0.046). Similarly, the odds were 9.56 when persons with an unknown source were compared with those with a healthcare source (95% CI 1.76–51.93; p<0.009). Acquiring MRSA from another (other) source compared with healthcare acquisition also had increased odds, but this result was not significant (OR 4.3, 95% CI 0.55–33.56; p<0.164).
Models 2–4 build on model 1 (the base model) by adding in the logs of pig, cattle, and veal calf densities per municipality, respectively, with the same individual level variables used in model 1 (Table 4). Model 2 builds on model 1 by adding a term for the log of pig density. The odds ratio comparing LA-MRSA to T-MRSA for a 1 log increase in pig density per hectare after adjusting for the individual risk factors (the variables in model 1) for LA-MRSA was 1.37 (95% CI 1.01–1.87, p<0.041). A doubling of the pig density per municipality increases the odds of LA-MRSA carriage compared with T-MRSA carriage by 24.7% after adjustment for individual level risk factors. Model 3 builds on model 1 by incorporating the log of the cow density per municipality. Adjusting for the individual level predictors, a 1 log increase in cow density yields a 2.28 increase in odds for LA-MRSA compared with T-MRSA (95% CI 1.17–4.45, p<0.016). Here, a doubling of cow density in a municipality increased the odds of LA-MRSA compared with T-MRSA by 76.9%. The odds ratio of carrying LA-MRSA compared with those of carrying T-MRSA in model 4 for a 1 log increase in veal calf density after adjustment for individual variables was 1.37 (95% CI 1.08–1.72, p<0.009). Thus, a doubling of the veal calf density per municipality yields a 24.09% increase in the odds of carrying LA-MRSA compared with carrying T-MRSA.
The Hosmer-Lemeshaw goodness-of-fit tests indicate that all models fit the data sufficiently well. The Akaike information criteria and likelihood ratio values for models 2–4 indicate that adding area-level animal density variables improves the original model (model 1). Variograms of residuals from the 4 models did not reveal any substantial spatial variation.
Cluster Detection
Cluster detection analysis results indicate that after adjusting for the size of the population in a given municipality, 1 significant cluster of LA-MRSA cases (relative risk 5.2, p<0.014) was found when a maximum of 20% of the population at risk was designated as the maximum spatial cluster size. Figure 3 (panels A–C) shows the cluster detection results mapped on top of veal calf density, all cattle density, and pig density for visual identification of associations.
To test whether accounting for livestock density at the municipality level would eliminate the existence of this hot spot of LA-MRSA, we ran additional analyses in SaTScan with adjustment for the density of each animal population separately (28). These results indicated that adjusting for animal densities eliminated the presence of the cluster, further supporting the hypothesis that livestock densities per municipality are key risk factors for LA-MRSA carriage.
Our findings indicate that regional density of livestock is a notable risk factor for nasal carriage of LA-MRSA for persons with and without direct contact with livestock. This finding has been emphasized in recent research that found LA-MRSA carriage in persons without connections to the farm environment (29). A recent study indicated that proximity to farms is a potential risk factor, even in absence of direct contact between humans and animals (30). In addition, MRSA has been found in meat; diet may therefore provide another route of exposure for the general population (31,32).
We observed in the multivariate analysis that living in a region with high cattle density conferred higher odds of LA-MRSA carriage than did living in a region of high pig or veal calf density. We are not certain what may explain this association, but it does warrant further investigation. We found in our multivariate models that some of the risk factors previously identified by univariate analysis by van Loo and colleagues (8) dropped out as being less significant when regional livestock density was included in a multivariate model, such as direct contact with pigs and cows and living in a rural location. Intriguingly, acquiring MRSA from an “unknown” source remained highly significant in all of the multivariate models. These results highlight the value of considering these individual-level variables, together with regional level data, as an update of the univariate analysis conducted by van Loo and colleagues in 2007.
This analysis is limited by the small size of the dataset. However, even with such a small dataset, and after adjusting for known and supposed LA-MRSA risk factors, the densities of livestock per municipality remain strong and independent risk factors for LA-MRSA carriage.
A second limitation of the study is that the case-patients were initially restricted to index case-patients, which inherently selected against detecting secondary transmission. Conducting a future study that includes non–index case-patients would produce a more accurate picture.
A final limitation is the possibility of recall bias in the participants’ reports of exposures to livestock, leading to a misclassification of exposure. Such a nondifferential information bias may have biased our results toward the null hypothesis.
This work has potential policy implications for MRSA surveillance in countries where a substantial percentage of total MRSA cases are LA-MRSA, such as the Netherlands. Starting in 2006, health policy in the Netherlands has required testing for MRSA carriage on admission to the hospital for persons living or working on pig farms. This study suggests that this screening program may need to be expanded to include other persons from municipalities where livestock densities are high.
Although research has indicated that LA-MRSA is not readily transmitted from person to person (20,33), cases continue to be reported with no identified livestock-associated risk factors. Some possible modes of exposure could involve contact with other domesticated animals, person-to-person contact, and contact with contaminated meat or, in some cases, environmental pathways such as air or waste releases from farms to the surrounding community. Future research should assess these factors in terms of their relationship to living in livestock-dense areas and the likelihood of exposure to MRSA with a larger sample sizes. Information from the statistically significant cluster in the cluster detection analysis can be used to target interventions in the Netherlands. Future work should investigate more recent cases, specifically those without direct links to livestock farming.
We confirm what has been suggested in other studies that veal calf farming (not just pig farming) is a risk factor for LA-MRSA. We also demonstrate a relationship between nontypeable MRSA and all cattle, not just veal calves. The hypothesis that a relationship exists between other types of cattle farming and LA-MRSA carriage should also be explored in further research.
These findings also have the potential to affect countries beyond the Netherlands. Although pig farming is an important industry in the Netherlands, its scale there is greatly overshadowed by the density of pig-farming operations in the United States. In the United States, in 2007, there were 75,442 pig farms, 8,206 of which have >2,000 pigs on them (10.9%) (34). For comparison, in the Netherlands in 2000, of the 14,524 pig farms, only 983 housed >2,000 swine (6.8%). Future work could investigate the relationship between these more intensive livestock operations and drug-resistant microorganisms, especially LA-MRSA, which at present has not been widely detected in the United States. These research findings will be useful for generating hypotheses regarding the epidemiology of LA-MRSA in the Netherlands and can provide a warning that where one lives may play a critical role in one’s risk of disease.
Dr Feingold is the Glenadore and Howard L. Pim postdoctoral fellow in global change at Johns Hopkins University, Department of Earth and Planetary Sciences. She is also an affiliate at the Duke Global Health Institute at Duke University in Durham, North Carolina. She uses spatial epidemiologic methods to examine the relationship between land use and public health.
Acknowledgment
We thank the Johns Hopkins Center for a Livable Future and the Pew Charitable Trusts for funding this project.
References
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–82. DOIPubMedGoogle Scholar
- European Center for Disease Prevention and Control. The bacterial challenge: time to react. Stockholm (Sweden): The Center; 2009.
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. DOIPubMedGoogle Scholar
- Kluytmans-VandenBergh MF, Kluytmans JA. Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clin Microbiol Infect. 2006;12(Suppl 1):9–15. DOIPubMedGoogle Scholar
- Silbergeld EK, Davis M, Leibler JH, Peterson AE. One reservoir: redefining the community origins of antimicrobial-resistant infections. Med Clin North Am. 2008;92:1391–407. DOIPubMedGoogle Scholar
- Fitzgerald JR. Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol. 2012;20:192–8. DOIPubMedGoogle Scholar
- van Loo I, Huijsdens X, Tiemersma E, de Neeling A, van de Sande-Bruinsma N, Beaujean D, Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis. 2007;13:1834–9. DOIPubMedGoogle Scholar
- Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis. 2005;11:1965–6. DOIPubMedGoogle Scholar
- Haenen A, Huijsdens X, Pluister C, van Luit M, van Luit T, Bosch T, Surveillance van MRSA in Nederland in 2008. Infectieziekten Bulletin. 2010;21:162–9. [cited 2012 Sep 12]. http://www.rivm.nl/dsresource?objectid=rivmp:55106&type=org&disposition=inline
- Graveland H, Wagenaar JA, Bergs K, Heesterbeek H, Heederik D. Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS ONE. 2011;6:e16830. DOIPubMedGoogle Scholar
- Huijsdens XW, Bosch T, van Santen-Verheuvel MG, Spalburg E, Pluister GN, van Luit M, Molecular characterisation of PFGE non-typable methicillin-resistant Staphylococcus aureus in The Netherlands, 2007. Euro Surveill. 2009;14:19335.PubMedGoogle Scholar
- Van Cleef BA, Broens EM, Voss A, Huijsdens XW, Zuchner L, Van Benthem BH, High prevalence of nasal MRSA carriage in slaughterhouse workers in contact with live pigs in The Netherlands. Epidemiol Infect. 2010;138:756–63. DOIPubMedGoogle Scholar
- Graveland H, Wagenaar JA, Heesterbeek H, Mevius D, van Duijkeren E, Heederik D. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS ONE. 2010;5:e10990. DOIPubMedGoogle Scholar
- Van Den Broek IVF, Van Cleef BAGL, Haenen A, Broens EM, Van Der Wolf PJ, Van Den Broek MJM, Methicillin-resistant Staphylococcus aureus in people living and working in pig farms. Epidemiol Infect. 2009;137:700–8. DOIPubMedGoogle Scholar
- Wertheim HF, Vos MC, Boelens HA, Voss A, Vandenbroucke-Grauls CM, Meester MH, Low prevalence of methicillin-resistant Staphylococcus aureus (MRSA) at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J Hosp Infect. 2004;56:321–5. DOIPubMedGoogle Scholar
- Catry B, Van Duijkeren E, Pomba MC, Greko C, Moreno MA, Pyorala S, Reflection paper on MRSA in food-producing and companion animals: epidemiology and control options for human and animal health. Epidemiol Infect. 2010;138:626–44. DOIPubMedGoogle Scholar
- van Cleef BA, Graveland H, Haenen AP, van de Giessen AW, Heederik D, Wagenaar JA, Persistence of livestock-associated methicillin-resistant Staphylococcus aureus in field workers after short-term occupational exposure to pigs and veal calves. J Clin Microbiol. 2011;49:1030–3. DOIPubMedGoogle Scholar
- Köck R, Loth B, Köksal M, Schulte-Wülwer J, Harlizius J, Friedrich AW. Does nasal colonization with livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) in pig farmers persist after holidays from pig exposure? Appl Environ Microbiol. 2012;78:4046–7. DOIPubMedGoogle Scholar
- van Cleef BA, Verkade EJ, Wulf MW, Buiting AG, Voss A, Huijsdens XW, Prevalence of livestock-associated MRSA in communities with high pig-densities in The Netherlands. PLoS ONE. 2010;5:e9385. DOIPubMedGoogle Scholar
- ESRI Inc. ArcGIS 9.3. Redlands, CA; 1999–2008.
- Google earth (5.2.1.1588). [cited 2010 Ag 11]. http://www.google.com/earth/index.html
- StataCorp. Stata statistical software: release 10. College Station (TX): StataCorp LP; 2007.
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2010.
- Waller L, Gotway C. Applied spatial statistics for public health data. New York: Wiley; 2004.
- University of California, Los Angeles, Academic Technology Services. FAQ: How do I interpret a regression model when some variables are log transformed? 2008 [cited 2011 May 2]. http://www.ats.ucla.edu/stat/mult_pkg/faq/general/log_transformed_regression.htm
- Kulldorf M. SaTScan—software for the spatial, temporal and space-time scan statistics. 2006. [cited 2010 Oct 25]. http://www.satscan.org
- Moritz ED, Smith TC. Livestock-associated Staphylococcus aureus in childcare worker. Emerg Infect Dis. 2011;17:742–4. DOIPubMedGoogle Scholar
- van Cleef BA, Monnet DL, Voss A, Krziwanek K, Allerberger F, Struelens M, Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg Infect Dis. 2011;17:502–5. DOIPubMedGoogle Scholar
- O’Brien AM, Hanson BM, Farina SA, Wu JY, Simmering JE, Wardyn SE, MRSA in conventional and alternative retail pork products. PLoS ONE. 2012;7:e30092. DOIPubMedGoogle Scholar
- van Loo IH, Diederen BM, Savelkoul PH, Woudenberg JH, Roosendaal R, van Belkum A, Methicillin-resistant Staphylococcus aureus in meat products, the Netherlands. Emerg Infect Dis. 2007;13:1753–5. DOIPubMedGoogle Scholar
- Köck R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill. 2010;15:19688.PubMedGoogle Scholar
- National Agricultural Statistics Service/US Department of Agriculture. Census of agriculture, 2002, 2007. [cited 2009 April 5]. http://www.agcensus.usda.gov/index.php
Figures
Tables
Cite This ArticleTable of Contents – Volume 18, Number 11—November 2012
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Beth J. Feingold, Johns Hopkins University–Earth and Planetary Sciences, 3400 Charles St, 301 Olin Hall Baltimore, MD 21218, USA
Top