Volume 18, Number 11—November 2012
Research
Mycoplasmosis in Ferrets
Cite This Article
Citation for Media
Abstract
We report an outbreak of severe respiratory disease associated with a novel Mycoplasma species in ferrets. During 2009–2012, a respiratory disease characterized by nonproductive coughing affected ≈8,000 ferrets, 6–8 weeks of age, which had been imported from a breeding facility in Canada. Almost 95% became ill, but almost none died. Treatments temporarily decreased all clinical signs except cough. Postmortem examinations of euthanized ferrets revealed bronchointerstitial pneumonia with prominent hyperplasia of bronchiole-associated lymphoid tissue. Immunohistochemical analysis with polyclonal antibody against Mycoplasma bovis demonstrated intense staining along the bronchiolar brush border. Bronchoalveolar lavage samples from 12 affected ferrets yielded fast-growing, glucose-fermenting mycoplasmas. Nucleic acid sequence analysis of PCR-derived amplicons from portions of the 16S rDNA and RNA polymerase B genes failed to identify the mycoplasmas but showed that they were most similar to M. molare and M. lagogenitalium. These findings indicate a causal association between the novel Mycoplasma species and the newly recognized pulmonary disease.
The number of pet ferrets in the United States has grown rapidly, from an estimated 800,000 in 1996 (1) to an estimated 7–10 million in 2007 (2). Also in the United States, ferrets have become the third most common household pet; their popularity as a pet in Europe is similar (3). The common respiratory diseases in pet ferrets are caused by viruses; canine distemper is probably the most virulent (4). Ferrets also are highly susceptible to human influenza virus, but disease is rarely severe (5,6). Bacteria rarely cause disease outbreaks in ferret populations, but they do cause disease in individual ferrets (7–9).
In 2007, in the state of Washington, USA, an outbreak of respiratory disease characterized by a dry, nonproductive cough was observed in 6- to 8-week-old ferrets at a US distribution center of a commercial pet vendor (Video). Over a 4-year period, ≈8,000 ferrets, equal numbers of both sexes, were affected. Every 2–3 weeks, kits had been shipped in groups of 150–200 from a commercial breeding facility in Canada to the distribution center. At 5 weeks of age, before shipment to the distribution center, each kit received a single vaccination for distemper (DISTEM R-TC; Schering Plough, Kenilworth, NJ, USA).
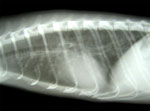
Some ferrets exhibited hemoptysis, labored breathing, sneezing, and conjunctivitis. Almost 95% of the ferrets were affected, but almost none died. Symptomatic ferrets were selected from each shipment for testing; results of heartworm screening, PCR and serologic testing for distemper, and serologic testing for influenza virus were negative. Cytologic examination of bronchioalveolar lavage (BAL) samples yielded few inflammatory cells. Thoracic ultrasonography found no abnormalities. Thoracic radiographs showed a mild bronchointerstitial pattern with peribronchial cuffing (Figure 1). Complete blood counts and chemistry results were within reference ranges (10,11).
Affected ferrets received broad spectrum antimicrobial drugs, bronchodilators, expectorants, nonsteroidal anti-inflammatory drugs, and nebulization; all clinical signs except the dry cough temporarily decreased. Numerous ferrets from the distribution center were later surrendered to a ferret rescue and shelter operation, where their cough continued for as long as 4 years.
Affected Ferrets
In April 2009, a 2-year-old, spayed female ferret at the ferret rescue and shelter, which had originated from the breeding facility in Canada and passed through the US distribution center, became acutely dyspneic and died within 15 minutes. The ferret had shown signs of respiratory disease since arrival at the shelter. Previously at the shelter, 2 ferrets, 4.5 years of age, had shown chronic cough; 1 died of dyspnea in October 2010, and the other was euthanized for humane purposes in November 2010. Both had originated from the breeding facility and passed through the distribution center. Postmortem examinations were performed on all 3 ferrets. After the 2-year-old ferret died in April 2009, BAL samples and ocular swabs were obtained in July 2009 from 3 other ferrets with a history of respiratory disease since their arrival at the ferret shelter. For further diagnostic investigation, BAL samples and ocular swabs were collected from 9 additional affected ferrets in January 2010; one of these was the ferret that died in October 2010.
Survey of Healthy Ferrets
At a large commercial breeding facility in which signs of respiratory disease had not been observed, BAL samples were obtained from 10 euthanized healthy male ferrets, 5 weeks to 5 years of age. Before postmortem examination, samples were collected from the euthanized ferrets by BAL through an incision in the caudal trachea. Nonbacteriostatic saline (10 mL/kg) was flushed into the caudal trachea and lungs and then recovered by aspiration into the syringe. That process was repeated 2× and the final flush fluid was submitted for bacterial culture. Complete postmortem examinations were performed, and sections of lung were collected for bacterial and mycoplasma culture. Additional tissue samples were collected from the lungs, trachea, nasal turbinates, brain, liver, kidneys, spleen, stomach, small and large intestine, thoracic and mesenteric lymph nodes, pancreas, and adrenal glands for routine histopathologic examination.
Histologic and Immunohistochemical Analyses and Confocal Microscopy
From the 3 ferrets that died April 2009–November 2010, postmortem tissue samples (lungs, trachea, nasal turbinates, brain, liver, kidneys, spleen, stomach, small and large intestine, thoracic and mesenteric lymph nodes, pancreas, and adrenal glands) were collected. They were fixed in neutral-buffered, 10% formalin solution and processed by standard methods for histopathologic examination.
For immunohistochemical examination, paraffin-embedded samples of lung from the 3 ferrets that died were cut into 5-μm sections. An Enhanced Alkaline Phosphatase Red Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ, USA) and bulk buffers specifically designed for use on the BenchMark Automated Staining System (Ventana Medical Systems, Inc.) were used for immunolabeling. Slides were baked in a drying oven at 60°C for 20 min, barcode labeled, and placed in the BenchMark for deparaffinization and heat-induced epitope retrieval. Slides were then incubated with a mouse monoclonal antibody against mycoplasma (primary antibody) (Chemicon, Billerica, MA, USA) at a concentration of 1:100 for 30 min. The monoclonal antibody was raised against M. bovis strain M23, but it is known to cross-react with numerous other mycoplasma species.
The slides were counterstained by using hematoxylin (Ventana Medical Systems, Inc.), then dehydrated, cleared, and mounted. For a positive control, we used formalin-fixed, paraffin-embedded sections of lung from an M. bovis–positive cow (tested by bacterial culture). For negative controls, we replaced the primary antibody with homologous nonimmune serum.
A Zeiss 510 microscope (Jena, Germany) was used for confocal imaging to acquire fluorescent images, and the Zeiss LSM image analysis software was used for characterizations. The images represented a differential interference contrast/Nomarski image with green (488 nm, argon laser excitation, fluorescein isothiocyanate [FITC]) and red (543 nm, rhodamine, helium–neon excitation, tetramethylrhodamine-5- [and 6-] isothiocyanate [TRITC])–labeled overlay to demonstrate localization of labels, as described, with slight modified according to Ubels et al. (12).
Transmission and Scanning Electron Microscopy
For transmission electron microscopy, lung tissue samples that had been fixed in neutral-buffered, 10% formalin solution were trimmed into 2-mm pieces and postfixed in 1% osmium tetroxide in 0.1 M sodium phosphate buffer for 2 h. Tissues were serially dehydrated in acetone and embedded in Poly/Bed 812 resin (Polysciences Inc., Warrington, PA, USA) in flat molds. Sections were obtained with a Power Tome XL ultramicrotome (Boeckeler Instruments, Tucson, AZ, USA). To identify areas of interest, we stained semithin (0.5-μm) sections with epoxy tissue stain and examined them under a light microscope. Then we cut ultrathin (70-nm) sections, mounted them onto 200-mesh copper grids, stained them with uranyl acetate and lead citrate, and examined them under a 100 CXII transmission electron microscope (JEOL, Peabody, MA, USA).
For scanning electron microscopy, formalin-fixed lung tissues were trimmed into 2–4-mm pieces, postfixed for 1 h in 1% osmium tetroxide, and rinsed for 30 min in 0.1 M sodium phosphate buffer. Tissues were serially dehydrated in ethanol and dried in a critical point dryer (Model 010; Balzers, Witten, Germany) with liquid carbon dioxide as the transitional fluid. Samples were mounted on aluminum studs by using carbon suspension cement (SPI Supplies, West Chester, PA, USA). Samples were then coated with an osmium coater (NEOC-AT; Meiwa Shoji Co., Tokyo, Japan) and examined in a JSM-7500F (cold field emission electron emitter) scanning electron microscope (JEOL).
Bacterial Cultures
We submitted 12 BAL samples and 12 ocular swab samples for bacterial and mycoplasma culture by standard microbiologic techniques. The samples were from live ferrets that originated from the distribution center and showed clinical signs of respiratory disease, including coughing. We also submitted 10 BAL samples from 10 healthy ferrets from a different commercial breeding facility not affected by respiratory disease.
PCR and Sequence Analysis
Only mycoplasmas obtained from BAL samples were analyzed by PCR and nucleic acid sequencing. A plug of agar containing Mycoplasma spp. colonies was gouged from the surface of a mycoplasma agar plate by using a 10-μL disposable inoculation loop and transferred to a microcentrifuge tube. The agar plug was digested by addition of 200 μL of Buffer ATL (QIAGEN, Valencia, CA, USA) and 20 μL of proteinase K solution (QIAGEN), followed by overnight incubation at 56°C. DNA was extracted from the digest by using a DNeasy Blood and Tissue kit (QIAGEN) according to manufacturer’s instructions.
For PCR, we used 2 sets of primers selective for the bacterial 16S rDNA or the mycoplasma RNA polymerase B (rpoB) gene. The nucleic acid sequences for the 16s rDNA gene were 5′-AGAGTTTGATCMTGGCTCAG-3′ for the forward primer and 5′-GGGTTGCGCTCGTTR-3′ for the reverse primer; this primer set produced an amplicon of ≈1,058 bp. The nucleic acid sequences for the mycoplasma rpoB gene were 5′- GGAAGAATTTGTCCWATTGAAAC-3′ for the forward primer and 5′- GAATAAGGMCCAACACTACG-3′ for the reverse primer; this primer set produced an amplicon of ≈1,613 bp. The PCRs were performed by using Platinum Taq DNA Polymerase High Fidelity (Invitrogen Corp., Carlsbad, CA, USA). The reaction mixture consisted of 3 μL DNA; 1 unit of Platinum Taq DNA Polymerase High Fidelity; 60 mmol/L Tris-SO4 (pH 8.9); 18 mmol/L ammonium sulfate; 2 mmol/L magnesium sulfate; 0.2 mmol/L each of dATP, dCTP, dGTP and dTTP; 16.9 μL molecular biology grade water; and 0.5 µmol/L each of the PCR primer. The reaction conditions for the 16s rDNA gene were 1 cycle at 94°C for 4 min; 35 cycles at 94°C for 30 s, 58°C for 45 s, 68°C for 75 s; followed by a final extension step at 68°C for 5 min. The reaction conditions for the rpoB gene were 1 cycle at 94°C for 4 min; 40 cycles at 94°C for 45 s, 55°C for 45 s, 68°C for 90 s; followed by a final extension step at 68°C for 5 min.
The PCR products were stained with ethidium bromide and examined after electrophoresis through a 1.5% agarose gel. The PCR amplicons were excised from gels, purified by using the QIAquick Gel Extraction Kit (QIAGEN), and submitted to the Research Technology Support Facility at Michigan State University for nucleic acid sequencing. Several internal primers were designed to derive the complete sequences of the PCR amplicons. The derived sequences were edited by using Sequencher software (Gene Codes Corporation, Ann Arbor, MI, USA) and analyzed by using BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi).
The nucleic acid sequences of the mycoplasma isolates and sequences from other Mycoplasma spp. obtained from GenBank were imported into the MEGA4 program (www.megasoftware.net), aligned by using ClustalW in the MEGA4 program, and subjected to phylogenetic analyses. For each isolate analyzed, 933 bp of the 16S rDNA gene sequence and 733 bp of the rpoB gene sequence were available. Phylogenetic trees were constructed by using the neighbor-joining method; data were resampled 1,000× to generate bootstrap percentage values.
Gross and Histologic Lesions
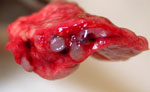
Gross and histologic lesions from the 3 ferrets that died or were euthanized because of respiratory disease were similar and restricted to the lungs. The lungs were characterized by multifocal, tan to gray, somewhat firm nodules centered on airways randomly distributed throughout the pulmonary parenchyma (Figure 2). Hematoxylin and eosin–stained lung sections revealed a moderate bronchointerstitial pneumonia with severe bronchiole-associated lymphoid tissue (BALT) hyperplasia (Figure 3, panel A). BALT hyperplasia was commonly associated with marked narrowing of airway lumina. Additional findings included moderate perivascular lymphoid cuffing and diffuse pulmonary congestion. The lumina of some bronchi contained large amounts of mucus admixed with few sloughed epithelial cells and lymphocytes (catarrhal bronchitis).
Immunohistochemical examination (with antibodies against mycoplasmas) of affected lung tissue from all 3 ferrets that died exhibited strong labeling along the brush border of terminal respiratory epithelial cells (Figure 3, panel B). There was no penetration of organisms into the adjacent pulmonary parenchyma. With the same antibodies against mycoplasmas labeled with a fluorescent chromogen, confocal laser microscopy showed positive labeling along the apical border of the lining epithelium of terminal airways (Figure 3, panel C). Additional immunohistochemical examination and reverse transcription PCR for canine distemper and influenza A viruses, performed on samples of lung from all 3 ferrets that died, detected no virus.
Transmission electron microscopy showed bronchial epithelial cells with loss of cilia and cellular degeneration characterized by swelling of endoplasmic reticulum, vacuolization of mitochondria with loss of christae, and intranuclear chromatin dispersement. Attached to the apical surface of a ciliated cell were pleomorphic, round to ovoid, ≈0.8-µm mycoplasma-like organisms (Figure 4).
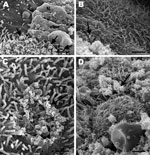
Electron microscopy showed severe denudation of bronchial epithelial cells. Cilia were commonly lost or had undergone degenerative changes characterized by bulbous swelling (Figure 5, panel A). Many necrotic bronchial epithelial cells were adhered to the luminal surface, and many pleomorphic mycoplasma-like organisms were diffusely attached to the mucosal surface of bronchi and bronchioles (Figure 5, panel B). In some areas, focal loss of cilia and cell membrane damage and mycoplasma-like organisms were observed along the periphery of such lesions (Figure 5, panel C). In other areas, the mucosal surface was covered by many mycoplasma-like organisms that completely obscured the cilia (Figure 5, panel D). Among the 10 healthy ferrets, no gross or histologic lesions suggestive of mycoplasma infection were identified.
Bacteria
The 12 BAL samples from affected ferrets were all positive for fast-growing, glucose-fermenting mycoplasmas but negative for other bacteria. Ocular swabs from these ferrets were negative for bacteria. No bacteria or mycoplasmas were isolated from the 10 healthy ferrets.
PCR and Sequences
Analyses of nucleic acid sequences from the 16S rDNA gene (GenBank accession nos. JQ910955– JQ910966) for each of the 12 mycoplasma isolates showed that the isolates were 99% similar to each other and segregated the isolates into 2 groups defined by nucleotide differences at 3 positions. Phylogenetic analysis with partial 16S rDNA gene sequences showed that the isolates were 96% to 97% similar to M. molare (isolated from a canid). Other closely related Mycoplasma spp. included M. lagogenitalium (isolated from Afghan pika), M. neurolyticum (isolated from mice and rats), M. sp. LR5794 (isolated from raccoons), M. collis (isolated from mice and rats), M. cricetuli (isolated from Chinese hamsters), and M. sp. EDS (isolated from house musk shrews) (Figure 6, panel A). On the basis of the 16S rDNA gene sequences, these mycoplasmas isolated from ferrets, along with the aforementioned closely related Mycoplasma spp., are in the hominis group of mycoplasmas.
Analyses of nucleic acid sequences from the rpoB gene (GenBank accession nos.. JQ91067–JQ91078) for each of the mycoplasma isolates from ferrets segregated the isolates into 2 groups of genetic variants (groups 1 and 2), which were 90% –91% similar to each other. Within a group, the isolates were 99%–100% or 98%–100% similar to each other. Although nucleotide differences were identified in as many as 12 positions within a group and 65 positions between groups, the corresponding amino acid sequences were 100% similar within a group and differed at only 2 aa positions between groups. Phylogenetic analysis showed that the partial rpoB gene sequences of the isolates were only 85%–86% similar with M. molare and 84%–86% similar to M. lagogenitalium, the most closely related Mycoplasma species. (Figure 6, panel B). Grouping of the isolates according to sequences of 16S rDNA and rpoB gene were in agreement for all but 1 isolate. Phylogenetic relatedness of these newly identified mycoplasmas to other Mycoplasma spp. was similar for the 16s rDNA and the rpoB genes.
Mycoplasma spp. are the smallest free-living prokaryotic microorganisms of the class Mollicutes (16). They lack a cell wall and are thought to have developed from genome reduction of gram-positive bacteria (17). Most species are host-specific facultative anaerobes and do not usually replicate in the environment (18). Their complex growth requirements include cholesterol, fatty acids, and amino acids (19). In the respiratory tract, mycoplasmas attach to ciliated epithelial cells by surface-exposed adhesions (20). Although the pathogenesis of host cell injury remains largely unknown, proposed virulence mechanisms include induction of proinflammatory cytokines by phagocytes (21), oxidative damage to host cells by production of toxic by-products (22), and cleavage of host DNA through nucleases (23). Many mycoplasmas cause B lymphocytes and/or T lymphocytes to commence dividing in a nonspecific manner (24). This mitogenic effect probably explains the characteristic BALT hyperplasia observed in infected host tissues. A commonly described strategy for immune evasion is phenotype plasticity, whereby reversible switching or modification of membrane protein antigens results in altered surface antigens (25). This mechanism might support the persistent, chronic nature of mycoplasmosis often observed. The precise role of mycoplasmas in various host species is often difficult to interpret because certain mycoplasmas can be isolated from apparently healthy animals.
The data presented here describe a recently emerging respiratory disease of ferrets, characterized especially by high morbidity rates and a dry, nonproductive cough, associated with an infection by a novel Mycoplasma species. To our knowledge, no Mycoplasma species have been associated with clinical disease in ferrets or other mustelids. On the basis of limited sequence data, the isolated mycoplasmas most likely represent a novel Mycoplasma specie or species.
In 1982, a study from Japan reported isolation of a glucose-fermenting mycoplasma from the oral cavities of 81% of clinically healthy ferrets kept in a laboratory setting (26). This mycoplasma isolate was not antigenically related to any reference strains from dogs, cats, sheep, cattle, mice, raccoon dogs, or a Japanese badger. In 1983, similarly fast-growing, glucose-fermenting mycoplasmas were isolated from the lungs of healthy mink kits (1–2 months of age) in Denmark (27). This species was named M. mustelae. Because the Mycoplasma spp. isolates from healthy ferrets or mink were not genetically characterized, comparison with the isolates from ferrets with respiratory disease in this study was not possible. The Mycoplasma species isolated from affected ferrets showed the highest sequence similarity to M. molare and M. lagogenitalium. M. molare was first isolated in 1974 from the pharynx of dogs with mild respiratory disease (28). However, the pathogenicity of M. molare in dogs or other species remains speculative. M. lagogenitalium was first isolated in 1997 from prepucial samples from apparently healthy Afghan pikas (29).
The 3 mycoplasma isolates obtained from BAL samples from 3 ferrets in July 2009 were highly homogenous according to limited sequence data. All 3 isolates were included in the mycoplasma isolate group 2. Of note, only 3 of the 9 isolates obtained from BAL samples from 9 ferrets in January 2010 had the same partial rpoB amino acid sequence data as the previous isolates and were also included in the mycoplasma isolate group 2. In contrast, the partial rpoB sequence of 5 of the more recent isolates differed by 9%–10% from that of the previous isolates, and the isolates were identified as belonging to mycoplasma isolate group 1. Whether these differences represent multiple Mycoplasma species circulating through the ferret population or a genetic change of the original mycoplasma over time is uncertain, as is the virulence of each of the potential strains. Only 1 isolate was identified in the bronchoalveolar lavage sample from a ferret for which postmortem examination confirmed lesions consistent with a mycoplasma infection. Experimental reproduction with the different isolates is required to further elucidate the virulence of each putative novel mycoplasma.
Respiratory disease attributed to mycoplasma infections in cattle (30), pigs (31), poultry (32), mice, and rats has been well described (33). The clinical signs and microscopic lesions in ferrets with the emerging respiratory disease described here closely resembled signs and lesions described for pigs infected with M. hyopneumoniae (34), rats infected with M. pulmonis (35), and cattle infected with M. bovis (36). For all of these species, chronic pulmonary mycoplasmosis is characterized by lymphoplasmacytic perivascular cuffing and extensive BALT hyperplasia, as was observed in ferrets in this study. Furthermore, M. cynos (37) and an untyped Mycoplasma species (38) reportedly cause pulmonary lesions similar to those in dogs and cats, respectively.
The similarity between the pathologic changes in the ferrets and those in other species with mycoplasmal pneumonia highly supports a causal relationship between the pulmonary disease and the identified novel mycoplasma in these ferrets. In addition, mycoplasmas were the only bacterial pathogens recovered from the respiratory tract of diseased ferrets, there was no microscopic evidence of a viral disease, and immunohistochemical and reverse transcription PCR results for canine distemper and influenza A were negative. Furthermore, mycoplasmas were not detected in the sampled population of healthy domestic ferrets 5 weeks to 5 years of age.
Because mycoplasmas have been recovered from the respiratory tract of apparently healthy mustelids (26,27), other unknown factors might have predisposed the lungs of these ferrets to colonization. The severity of the clinical signs might have been exacerbated by infections with secondary bacteria, as commonly occurs in other species (30,31,33), and antimicrobial drug therapy might have prevented isolation of such bacteria. A concurrent viral disease seems unlikely because characteristic microscopic lesions were absent and common respiratory viral pathogens in ferrets were not identified. We speculate that the stress of shipment from the breeding facility to the distribution center might have resulted in the disease manifestation. To more fully elucidate pathogenicity and disease dynamics in this species, experimental reproduction of the respiratory disease in ferrets is necessary.
Dr Kiupel is a board certified veterinary anatomic pathologist and professor in the Department of Pathobiology and Diagnostic Investigation, College of Veterinary Medicine, Michigan State University. He is also section chief of anatomic pathology in the Diagnostic Center for Population and Animal Health. His main research interest is carcinogenesis and emerging infectious diseases.
Acknowledgment
We thank the staff of the Washington Ferret Rescue and Shelter and the Diagnostic Center for Population and Animal Health laboratory (in particular Lori Moon, Theresa Mosser, and Thomas Wood) for technical help. We thank the generous donors for their support of the Ferrethealth Advancment Fund at Michigan State University (www.ferrethealth.msu.edu), which enabled us to investigate this novel disease.
References
- Jurek RM. A review of national and California population estimates of pet ferrets. Sacramento (CA): California Department of Fish and Game, Wildlife Management Division, Bird and Mammal Conservation Program, Report no. 98–09; 1998 [cited 2011 Aug 12]. http://www.dfg.ca.gov/wildlife/nongame/nuis_exo/ferret/ferret.html
- American Ferret Association. No drugs approved for use in ferrets: passage of MUMS may make it easier to market drugs for ferrets [cited 2011 Aug 12]. http://www.ferret.org/news/052404c.htm
- Glass H. Marderdünste im Mietshaus. Der Spiegel. 1999;23:232–4.
- Von Messling V, Springfeld C, Devaux P, Cattaneo R. A ferret model of canine distemper virus virulence and immunosuppression. J Virol. 2003;77:12579–91. DOIPubMedGoogle Scholar
- Langlois I. Viral diseases of ferrets. Vet Clin North Am Exot Anim Pract. 2005;8:139–60. DOIPubMedGoogle Scholar
- Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol. 2002;76:4420–9. DOIPubMedGoogle Scholar
- Martínez J, Martorell J, Abarca ML, Olvera A, Ramis A, Woods L, Pyogranulomatous pleuropneumonia and mediastinitis in ferrets (Mustela putorius furo) associated with Pseudomonas luteola infection. J Comp Pathol. 2012;146:4-10 [cited 2011 Aug 12]. Epub 2011 May 24. http://www.sciencedirect.com/science/article/pii/S0021997511000570
- Kendrick RE. Ferret respiratory diseases. Vet Clin North Am Exot Anim Pract. 2000;2:453–64.
- Peltola VT, Boyd KL, McAuley JL, Rehg JE, McCullers JA. Bacterial sinusitis and otitis media following influenza virus infection in ferrets. Infect Immun. 2006;74:2562–7. DOIPubMedGoogle Scholar
- Schoemaker NJ. Ferrets, skunks and otters. In: Meredith A, Johnson-Delaney C, editors. BSAVA manual of exotic pets. 5th ed. Quedgeley, Gloucester (UK): British Small Animal Veterinary Association; 2010:127–38.
- Orcutt C, Malakoff R. Ferrets: cardiovascular and respiratory system disorders. In: Keeble E, Meredith A, editors. BSAVA manual of rodents and ferrets, Quedgeley, Gloucester (UK): British Small Animal Veterinary Association; 2009:282–90.
- Ubels JL , Hoffman HM, Srikanth S, Resau JH, Webb CP. Gene expression in rat lacrimal gland duct cells collected using laser capture microdissection: evidence for K+ secretion by duct cells. Invest Ophthalmol Vis Sci. 2006;47:1876–85. DOIPubMedGoogle Scholar
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;. DOIPubMedGoogle Scholar
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25.PubMedGoogle Scholar
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. DOIGoogle Scholar
- Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, Fleischmann RD, The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. DOIPubMedGoogle Scholar
- Rogers MJ, Simmons J, Walker RT, Weisburg WG, Woese CR, Tanner RS, Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985;82:1160–4. DOIPubMedGoogle Scholar
- Pitcher DG, Nicholas RAJ. Mycoplasma host specificity: fact or fiction? Vet J. 2005;170:300–6. DOIPubMedGoogle Scholar
- Fabricant CG, Fabricant J, Van Demark PJ. Studies on the nutritional and growth requirements of Mycoplasma gallisepticum. J Gen Microbiol. 1964;35:135–44. DOIPubMedGoogle Scholar
- Kaufmann A, Mühlradt PF, Gemsa D, Sprenger H. Induction of cytokines and chemokines in human monocytes by Mycoplasma fermentans–derived lipoprotein MALP-2. Infect Immun. 1999;67:6303–8.
- Pilo P, Vilei EM, Peterhans E, Bonvin-Klotz L, Stoffel MH, Dobbelaere D, A metabolic enzyme as a primary virulence factor of Mycoplasma mycoides subsp. mycoides small colony. J Bacteriol. 2005;187:6824–31. DOIPubMedGoogle Scholar
- Paddenberg R, Weber A, Wulf S, Mannherz HG. Mycoplasma nuclease able to induce internucleosomal DNA degradation in cultured cells possess many characteristics of eukaryotic apoptotic nucleases. Cell Death Differ. 1998;5:517–28. DOIPubMedGoogle Scholar
- Naot Y, Merchav S, Ben-David E, Ginsberg H. Mitogenic activity of Mycoplasma pulmonis. I. Stimulation of rat B and T lymphocytes. Immunology. 1979;36:399–406.PubMedGoogle Scholar
- Wise KS. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993;1:59–63. DOIPubMedGoogle Scholar
- Koshimizu K, Kotani H, Syukuda Y. Isolation of mycoplasmas from experimental ferrets (Mustela putorius). Jikken Dobutsu. 1982;31:299–302.PubMedGoogle Scholar
- Salih MM, Friis NF, Arseculeratne SN, Freundt EA, Christiansen C. Mycoplasma mustelae, a new species from mink. Int J Syst Bacteriol. 1983;33:476–9. DOIGoogle Scholar
- Rosendal S. Mycoplasma molare, a new canine mycoplasma species. Int J Syst Bacteriol. 1974;24:125–30. DOIGoogle Scholar
- Kobayashi H, Runge M, Schmidt R, Kubo M, Yamamoto K, Kirchhoff H. Mycoplasma lagogenitalium sp. nov., from the preputial smegma of Afghan pikas (Ochotona rufescens rufescens). Int J Syst Bacteriol. 1997;47:1208–11. DOIPubMedGoogle Scholar
- Maunsell FP, Woolums AR, Francoz D, Rosenbusch RF, Step DL, Wilson DJ, Mycoplasma bovis infections in cattle. J Vet Intern Med. 2011;25:772–83. DOIPubMedGoogle Scholar
- Jordan FT. Avian mycoplasma and pathogenicity—a review. Avian Pathol. 1975;4:165–74.PubMedGoogle Scholar
- Schoeb TR. Respiratory diseases of rodents. Vet Clin North Am Exot Anim Pract. 2000;3:481–96.PubMedGoogle Scholar
- Sarradell J, Andrada M, Ramírez AS, Fernández A, Gómez-Villamandos JC, Jover A, A morphologic and immunohistochemical study of the bronchus-associated lymphoid tissue of pigs naturally infected with Mycoplasma hyopneumoniae. Vet Pathol. 2003;40:395–404. DOIPubMedGoogle Scholar
- Whittlestone P, Lemcke RM, Olds RJ. Respiratory disease in a colony of rats II: isolation of Mycoplasma pulmonis from the natural disease, and the experimental disease induced with a cloned culture of this organism. J Hyg (Lond). 1972;70:387–407. DOIPubMedGoogle Scholar
- Gagea MI, Bateman KG, Shanahan RA, van Dreumel T, McEwen BJ, Carman S, Naturally occurring Mycoplasma bovis–associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagn Invest. 2006;18:29–40. DOIPubMedGoogle Scholar
- Chalker VJ, Owen WM, Paterson C, Barker E. Brooks H, Rycroft AN, Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150:3491–7. DOIPubMedGoogle Scholar
- Kirchner BK, Port CD, Magoc TJ, Sidor MA, Ruben Z Spontaneous bronchopneumonia in laboratory dogs infected with untyped Mycoplasma spp. Lab Anim Sci. 1990;40:625–8.PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 18, Number 11—November 2012
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Matti Kiupel, Diagnostic Center for Population and Animal Health, Michigan State University, 4125 Beaumont Rd, 152A, Lansing, MI 48910, USA
Top