Volume 18, Number 9—September 2012
CME ACTIVITY - Policy Review
Control of Fluoroquinolone Resistance through Successful Regulation, Australia
Figure 1
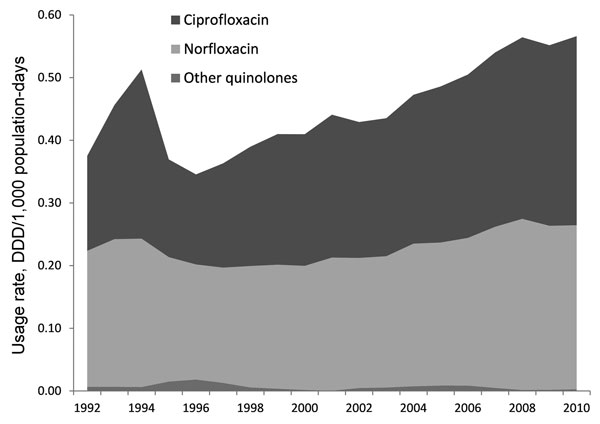
Figure 1. . . Data from Drug Utilization Sub-Committee Drug Utilization Database on Pharmaceutical Benefits Scheme and the Repatriation Pharmaceutical Benefits Scheme (RPBS) on subsidized medicines and estimates of non-subsidized medicines. RPBS data were calculated from continuous data on all prescriptions dispensed from a validated sample of community-based pharmacies. In-patient hospital prescribing is not included. Usage rate calculated on the basis of medication use of 1,000 persons per day. DDD, defined daily dose.
Page created: August 17, 2012
Page updated: August 17, 2012
Page reviewed: August 17, 2012
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.