Volume 19, Number 1—January 2013
Dispatch
Novel Epidemic Clones of Listeria monocytogenes, United States, 2011
Cite This Article
Citation for Media
Abstract
We identified a novel serotype 1/2a outbreak strain and 2 novel epidemic clones of Listeria monocytogenes while investigating a foodborne outbreak of listeriosis associated with consumption of cantaloupe during 2011 in the United States. Comparative analyses of strains worldwide are essential to identification of novel outbreak strains and epidemic clones.
In September 2011, the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, was notified of an increase of listeriosis cases linked to eating cantaloupe (1). The outbreak isolates were categorized into 4 pulsed-field gel electrophoresis (PFGE) profiles and serotypes 1/2a and 1/2b, the latter being seldom associated with large outbreaks (1,2). During August 2012, a fifth outbreak-associated subtype responsible for 1 case was detected, and CDC reported a final total of 147 cases from 28 US states, causing 33 deaths and 1 miscarriage (www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/index.html). The Food and Drug Administration (FDA) inspected the involved farm; outbreak strains matching 3 of the PFGE profiles from clinical samples were isolated from washed cantaloupes and various environmental surfaces within the facility (www.fda.gov/Food/FoodSafety/CORENetwork/ucm272372.htm#report).
Epidemic clones (ECs) of Listeria monocytogenes are defined as isolates of a presumably common ancestor that are genetically related and involved in different temporally and geographically unrelated outbreaks (2). Previously, multivirulence locus sequence typing (MVLST) accurately identified the 5 known ECs of L. monocytogenes, ECI–V (3,4). Also, comK prophage junction fragment (JF) sequences were demonstrated to be unique to EC strains of L. monocytogenes in individual facilities that processed ready-to-eat meat and poultry or in multiple plants manufacturing similar ready-to-eat products (5). The comK prophage may represent a rapid adaptation island that enables L. monocytogenes to rapidly adapt to and form biofilms in specific environmental niches (5).
Nine foodborne outbreak-associated isolates related to cantaloupe, representing the 4 outbreak strains initially identified, were selected for multilocus sequence typing (MLST) (6), MVLST (3), and comK prophage JF sequencing (5) to determine if they represented previously identified outbreak strains or known/novel ECs of L. monocytogenes (2–4). Isolates from cantaloupe samples were also compared with 29 US Department of Agriculture (USDA) isolates of L. monocytogenes retrieved from 2 US chicken processing plants (7,8).
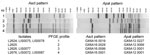
CDC confirmed identification of L. monocytogenes using the AccuProbe LISTERIA MONOCYTOGENES Culture Identification Test (Gen-Probe, San Diego, CA, USA) and by FDA according to the FDA Bacteriological Analytical Manual (www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/default.htm). Isolates were serotyped by using commercial antisera (Denka Seiken, Tokyo, Japan) and analyzed by PFGE (9) (Table; Figure 1). The Technical Appendix shows the relative distribution of the 4 PFGE profiles among clinical, food, or environmental samples.
Isolates were grown overnight in tryptic soy broth with yeast extract at 37°C, and DNA was extracted by using the Ultra Clean Microbial DNA Isolation Kit (Mo Bio Laboratories, Solana Beach, CA, USA) for isolates from CDC and USDA and the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) for isolates from FDA. Sequence types (STs) identified by using MLST were assigned as described (6) on the basis of whole genome sequence data (C. Tarr, Y. Chen, unpub. data) and compared with those publicly available (www.pasteur.fr/mlst). MVLST data were obtained as described (3) or extracted from whole genome sequences (Y. Chen, unpub. data). Sequences were compared with those on the MVLST database available in the laboratory of S.K. (3,4) and analyzed by using MEGA5.0 (10). New virulence types (VTs) were assigned to USDA isolates: VT60 (isolates 239, 441, 442, 458, 541, 565, 577); VT68 (350, 470); VT69 (247); VT70 (502); VT71 (450); VT72 (342), and VT73 (267). comK prophage JFs were sequenced as described (5). Prophage types (PTs) were assigned by comparing JF sequences with those available from previous reports (4,5). comK prophage JF sequences were submitted to GenBank for isolate L2676 (accession nos. JQ407079 and JQ407080) and 3 USDA isolates (accession nos. JQ750615–JQ750618).
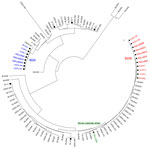
Isolates L2624, LIS0075, and LIS0078 (PFGE profile 1) belonged to the globally disseminated ST5 (6) and had the same VT (VT63) as 5 other 1/2b isolates in the database: isolates 10-0810 and 10-0811, from an imitation crabmeat–borne outbreak in Canada during 1996 (4,11); and isolates 98-0041, 233, and 466 (Table; Figure 2). Because VT63 isolates were associated with multiple outbreaks, they should be considered part of a novel EC (ECVI). ECVI isolates from cantaloupe and USDA isolate 233 showed no amplification of comK prophage JFs (Table). PT11/11 was identified during the 1996 imitation crabmeat–associated outbreak in Canada (4) and in USDA isolate 466 (Table). Further research is needed to determine why comK PTs were identical during different years and in different geographic locations and food processing plants.
Isolate L2625 (VT74, PFGE profile 2) from cantaloupe differed by 1 single nucleotide polymorphism in inlC from 3 other serotype 1/2a VT61 isolates (10-4758, 10-4754, and 06-6956) associated with the 2002 cheese-associated listeriosis outbreak in Canada (4,12) (Table; Figure 2). L2625 was assigned to ST29, an infrequent sequence type in the Institut Pasteur MLST database that differs from the ST (ST405) assigned to the isolates from cheese in the 2002 outbreak in Canada. No amplification of comK prophage JFs was observed, consistent with the PTs in the 2002 cheese-associated outbreak in Canada (4). Given this evidence, isolate L2625 does not represent a novel EC but should be considered a novel outbreak strain.
Isolates L2626 and LIS0077 (PFGE profile 3, ST7) and L2676, LIS0072, and LIS0087 (PFGE profile 4, ST561) from cantaloupe samples shared the same VT (VT56) as isolates 10-0813 and 10-0812 associated with a listeriosis outbreak related to whipping cream during 2000 in Canada (4,12) and isolates 06-6909, BL0047, 261, and 498 (Table; Figure 2). These Listeria isolates from cantaloupe displayed 2 highly similar PFGE profiles and STs, and the same serotype, ApaI PFGE pattern, and VT (Table; Figure 1). Isolates L2626 and LIS0077 showed no amplification of comK prophage JFs, which was also consistent with the upstream PT in the outbreak associated with whipping cream in Canada (Table). The JF sequences in isolates L2676, LIS0072, and LIS0087 were identical to those in USDA isolate 261 (Table). These isolates matched those from the whipping cream–associated outbreak in Canada in terms of VT56 and downstream PT (PT13) (Table). However, the upstream JF could not be amplified in the strain identified in whipping cream (4), possibly because of extensive recombination within the comK prophage (13), especially in the upstream JF (5). These STs and VTs were also found in clinical isolates over extended periods (6). Therefore, by definition (2,3), these isolates also represent a novel EC (ECVII).
Different clones, particularly ECVI and ECVII, might have cocolonized niches or harborage sites within the cantaloupe processing facility, possibly explaining the multiple strains associated with this outbreak. Serotype 4b L. monocytogenes strains, of the same genetic lineage as serotype 1/2b strains, reportedly survived and grew substantially better in mixed-serotype biofilms containing a specific strain of serotype 1/2a (14). Although a biofilm was not detected in the cantaloupe facility, because the facility had already been extensively cleaned and sanitized before FDA sampling, further research is needed to determine the potential for these strains to cocolonize with biofilms.
Six of the 7 currently identified ECs were found at some point in 1 or both of the US chicken processing plants included in the study (Figure 2). Listeriosis cases and outbreaks have been associated with consumption of undercooked raw chicken and ready-to-eat poultry products (2,4). Additional research is needed to determine whether poultry or poultry processing plants could be responsible for the global dissemination of ECs of L. monocytogenes.
The molecular epidemiology of L. monocytogenes strains involved in the 2011 multistate cantaloupe-associated outbreak was greatly enhanced by the use of subtyping markers with different levels of epidemiologic resolution. Particularly, MVLST enabled the detection of 1 novel 1/2a outbreak strain and 2 novel ECs of L. monocytogenes. In contrast to focusing on isolates from a single outbreak (15), our findings demonstrate that to detect new ECs it is important to analyze isolates from many sources around the world.
Dr Lomonaco is an assistant professor of Food Safety in the Department of Animal Pathology, Università degli Studi di Torino, Italy. Her main research interest is the development and application of molecular methods for subtyping L. monocytogenes.
Acknowledgments
We thank Rebecca Weinberg, Melissa Olsen-Rasmussen, and Lori Rowe for technical assistance and David Melka and Christine Keys for coordinating PFGE analysis and strain shipment. We also thank the members of the platform for Genotyping of Pathogens and Public Health at the Institut Pasteur for coding MLST alleles and profiles (available at www.pasteur.fr/mlst) and the Pennsylvania State University Genomics Core Facility staff for sequencing virulence gene and comK prophage junction fragment amplicons.
S.L. conducted this research while she was a visiting scientist at Pennsylvania State University during September 5–October 15, 2011. This study was partially funded by a USDA Special Grant on Milk Safety.
References
- Centers for Disease Control and Prevention. Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August–September 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1357–8.PubMedGoogle Scholar
- Cheng Y, Siletzky R, Kathariou S. Genomic divisions/lineages, epidemic clones, and population structure. In: Liu D, editor. Handbook of Listeria monocytogenes. Boca Raton (FL): CRC Press; 2008. p. 337–57.
- Chen Y, Zhang W, Knabel SJ. Multi-virulence-locus sequence typing identifies single nucleotide polymorphisms which differentiate epidemic clones and outbreak strains of Listeria monocytogenes. J Clin Microbiol. 2007;45:835–46. DOIPubMedGoogle Scholar
- Knabel SJ, Reimer A, Verghese B, Lok M, Ziegler J, Farber J, Sequence typing confirms that a predominant Listeria monocytogenes clone caused human listeriosis cases and outbreaks in Canada from 1988–2010. J Clin Microbiol. 2012;50:1748–51. DOIPubMedGoogle Scholar
- Verghese B, Lok M, Wen J, Alessandria V, Chen Y, Kathariou S, comK prophage junction fragments as markers for Listeria monocytogenes genotypes unique to individual meat and poultry processing plants and a model for rapid niche-specific adaptation, biofilm formation, and persistence. Appl Environ Microbiol. 2011;77:3279–92. DOIPubMedGoogle Scholar
- Ragon M, Wirth T, Hollandt F, Lavenir R, Lecuit M, Le Monnier A, A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008;4:e1000146. DOIPubMedGoogle Scholar
- Berrang ME, Meinersmann RJ, Frank JF, Smith DP, Genzlinger LL. Distribution of Listeria monocytogenes subtypes within a poultry further processing plant. J Food Prot. 2005;68:980–5 .PubMedGoogle Scholar
- Berrang ME, Meinersmann RJ, Frank JF, Ladely SR. Colonization of a newly constructed commercial chicken further processing plant with Listeria monocytogenes. J Food Prot. 2010;73:286–91 .PubMedGoogle Scholar
- Halpin JL, Garrett NM, Ribot EM, Graves LM, Cooper KL. Re-evaluation, optimization, and multilaboratory validation of the PulseNet-standardized pulsed-field gel electrophoresis protocol for Listeria monocytogenes. Foodborne Pathog Dis. 2010;7:293–8. DOIPubMedGoogle Scholar
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. DOIPubMedGoogle Scholar
- Farber JM, Daley EM, MacKie MT, Limerick B. A small outbreak of listeriosis potentially linked to the consumption of imitation crab meat. Lett Appl Microbiol. 2000;31:100–4. DOIPubMedGoogle Scholar
- Pagotto F, Ng L-K, Clark C, Farber J. Canadian listeriosis reference service. Foodborne Pathog Dis. 2006;3:132–7. DOIPubMedGoogle Scholar
- Orsi RH, Borowsky ML, Lauer P, Young SK, Nusbaum C, Galagan JE, Short-term genome evolution of Listeria monocytogenes in a non-controlled environment. BMC Genomics. 2008;9:539. DOIPubMedGoogle Scholar
- Pan Y, Breidt F Jr, Kathariou S. Competition of Listeria monocytogenes serotype 1/2a and 4b strains in mixed-culture biofilms. Appl Environ Microbiol. 2009;75:5846–52. DOIPubMedGoogle Scholar
- Laksanalamai P, Joseph LA, Silk BJ, Burall LS, Tarr CL, Gerner-Smidt P, Genomic characterization of Listeria monocytogenes strains involved in a multistate listeriosis outbreak associated with cantaloupe in US. PLoS ONE. 2012;7:e42448. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 19, Number 1—January 2013
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Stephen Knabel, Department of Food Science, The Pennsylvania State University, 405 Food Science Bldg, University Park, PA 16802, USA
Top