Volume 19, Number 10—October 2013
Dispatch
Safe Pseudovirus-based Assay for Neutralization Antibodies against Influenza A(H7N9) Virus
Figure
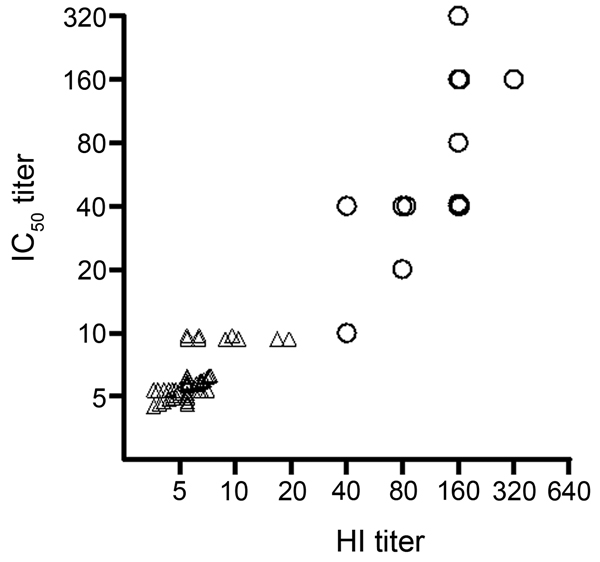
Figure. . Correlation between conventional hemagluttination (HI) titer and 50% inhibitory concentration (IC50) titer of pseudovirus-based assay for diagnosing influenza A(H7N9) virus infection. Correlation between the IC50 titer of the pseudovirus-based neutralization assay and the titer of conventional HI assay, tested with 14 serum samples collected >10 days after symptom onset from patients with real-time RT-PCR–confirmed 2013 influenza A(H7N9) infection (○) and 50 control samples (△, Spearman r = 0.88, p<0.0001, n = 64). To display the information from all the samples, overlapped markers were shifted on the x- and/or y-axis with small incremental units.
1These authors contributed equally to this article.
Page created: September 16, 2013
Page updated: September 16, 2013
Page reviewed: September 16, 2013
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.