Volume 19, Number 5—May 2013
Letter
Single Genotype of Anaplasma phagocytophilum Identified from Ticks, Camargue, France
Cite This Article
Citation for Media
To the Editor: Granulocytic anaplasmosis is a tickborne zoonosis caused by Anaplasma phagocytophilum bacteria, which are emerging in Europe. Besides infecting humans, A. phagocytophilum infect a wide range of wild and domestic mammals (1). In Europe, the Ixodes ricinus tick is the main vector for the bacteria, but A. phagocytophilum has also been detected in association with Rhipicephalus and Dermacentor spp. ticks (2). The climate and biotopes of the Mediterranean region are particularly favorable for several species of ticks and, therefore, for tickborne diseases.
Although I. ricinus ticks are rare or absent in the Mediterranean Basin, serosurveys performed on equine populations in Camargue, southern France, indicated an A. phagocytophilum seroprevalence of ≈10% (3). To investigate the prevalence and diversity of A. phagocytophilum bacteria in ticks in Camargue, we collected questing ticks from horse pastures and feeding ticks from horses.
Ticks feeding on horses were collected in randomly selected stables during 2007 (84 stables), 2008 (72 stable), and 2010 (19 stables). The stables were chosen among those where evidence of A. phagocytophilum seroconversion in horses had been previously found (3). In 2008 and 2010, questing ticks were collected by the dragging method in 19 pastures, around bushes, and in areas where horses spent the most time. Surveys were conducted in the spring, which represents the peak activity time of Ixodes ticks.
A total of 406 adult ticks were collected, representing 6 species: Rhipicephalus bursa, R. sanguineus, R. turanicus, R. pusillus, Dermacentor marginatus, and Hyalomma marginatum. Tick species were identified by morphologic criteria and molecular analyses based on mitochondrial 12S rDNA sequences (4). Total DNA was extracted from the ticks by using the NucleoSpin Tissue Kit (Macherey-Nagel, Düren, Germany) (5). A. phagocytophilum was detected by nested PCR targeting the 16S rDNA (Technical Appendix 1).
Of the 406 ticks, 40 were infected with A. phagocytophilum. The infected group included ticks from all 6 collected species except R. pusillus. Infection rates among the species ranged from 0 to 22% (Technical Appendix 2). The prevalence of A. phagocytophilum infection did not differ significantly between species (logistic regression model, p = 0.76) but was higher among questing ticks than feeding ticks (p<0.001; odds ratio 1.15).
We amplified 6 loci by nested PCR (Technical Appendix 1) to characterize A. phagocytophilum genetic diversity in positive samples: ankA, msp4, pleD, typA, and intergenic regions hemE–APH_0021 and APH_1099–APH_1100 (National Center for Biotechnology Information annotation). The GenBank accession numbers for the nucleotide sequences are JX197073–JX197368. No polymorphism was found among the 6 loci tested in the 40 A. phagocytophilum–positive ticks. The genotype identified was 100% identical to the reference sequence (NC_007797) for loci msp4, pleD, and typA and for intergenic regions hemE–APH_0021 and APH_1099–APH_1100. The ankA sequence was 96% similar (487 nt) to the reference sequence. The relevance of these loci as markers of diversity was verified (Technical Appendix 3).
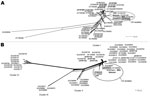
To study the phylogenetic relationships between cognate sequences, we included in our analysis all sequences available in GenBank for genes ankA and msp4. To account for recombination events that affect ankA and msp4 (data not shown) in phylogenetic analyses, we used Neighbor-Net networks (Figure). Phylogenetic analysis of msp4 (Figure, panel A) indicated that the genotype of A. phagocytophilum from ticks in Camargue was included in a clade that also includes genotypes that infect humans and horses in the United States.
The diversity of ankA sequences has been described as 4 phylogenetic clusters (6). All sequences obtained in our study were included in cluster I, particularly in a branch composed exclusively of sequences of A. phagocytophilum isolated from humans in the United States (Figure, panel B).
Previous studies investigating A. phagocytophilum have revealed a genetic diversity that is thought to have been caused by sympatric epidemiologic cycles involving different vectors and reservoir hosts (1,6,7). In 5 species of ticks (40 ticks total) that we collected from a 250-km2 area in southern France, we found only 1 genotype of A. phagocytophilum, which we determined to be phylogenetically close to genotypes found in the United States. Sequences phylogenetically related to bacteria in the United States were also observed in Sardinia (8) and Sicily (9).
The low diversity we found could be explained by a recent introduction of the bacteria into the area [although A. phagocytophilum–seropositive horses have been found in the area since 2001 (3)] or by a selective sweep linked to the particular ticks and host reservoir in Camargue. The 5 species of ticks that we found positive for A. phagocytophilum have been described as potential vectors of A. phagocytophilum in the Mediterranean Basin (2,10). Among the tick species in our investigation, R. bursa and R. sanguineus ticks are the 2 main carriers of A. phagocytophilum, and these ticks are likely to feed on humans and, thus, pose a risk of infection to the local population. Further studies are needed to address the potential effect of A. phagocytophilum–infected ticks on human health in this area and, more specifically, the relationship between genotype and pathogenicity.
Acknowledgment
We thank Curtis Nelson and Friederike von Loewenich for generously providing the DNA of strains HGE1, CRT, and Webster; Véronique Bachy for actively helping us to obtain samples from domestic animals; Magalie René-Martellet and Frédéric Beugnet for helping with the morphological identification of ticks; Gillian Martin for proofreading the manuscript; Nelly Dorr for creating the databases used in this study; and Françoise Rieu-Lesme and Sébastien Masséglia who were involved in laboratory work.
References
- Rikihisa Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin Microbiol Rev. 2011;24:469–89 . DOIPubMedGoogle Scholar
- de la Fuente J, Naranjo V, Ruiz-Fons F, Vicente J, Estrada-Peña A, Almazan C, Prevalence of tick-borne pathogens in ixodid ticks (Acari: Ixodidae) collected from European wild boar (Sus scrofa) and Iberian red deer (Cervus elaphus hispanicus) in central Spain. Eur J Wildl Res. 2004;50:187–96. DOIGoogle Scholar
- Leblond A, Pradier S, Pittel PH, Fortier G, Boireau P, Chadoeuf J, An epidemiological survey of equine anaplasmosis (Anaplasma phagocytophilum) in southern France [in French]. Rev Sci Tech. 2005;24:899–908 .PubMedGoogle Scholar
- Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. 2001;87:32–48 .PubMedGoogle Scholar
- Halos L, Jamal T, Vial L, Maillard R, Suau A, Le Menache A, Determination of an efficient and reliable method for DNA extraction from ticks. Vet Res. 2004;35:709–13 . DOIPubMedGoogle Scholar
- Scharf W, Schauer S, Freyburger F, Petrovec M, Schaarschmidt-Kiener D, Liebisch G, Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J Clin Microbiol. 2011;49:790–6. DOIPubMedGoogle Scholar
- Bown KJ, Lambin X, Ogden NH, Begon M, Telford G, Woldehiwet Z, Delineating Anaplasma phagocytophilum ecotypes in coexisting, discrete enzootic cycles. Emerg Infect Dis. 2009;15:1948–54. DOIPubMedGoogle Scholar
- Alberti A, Zobba R, Chessa B, Addis MF, Sparagano O, Parpaglia MLP, Equine and canine Anaplasma phagocytophilum strains isolated on the island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl Environ Microbiol. 2005;71:6418–22 . DOIPubMedGoogle Scholar
- de la Fuente J, Torina A, Caracappa S, Tumino G, Furla R, Almazan C, Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet Parasitol. 2005;133:357–62. DOIPubMedGoogle Scholar
- Toledo A, Olmeda AS, Escudero R, Jado I, Valcarcel F, Casado-Nistal MA, Tick-borne zoonotic bacteria in ticks collected from central Spain. Am J Trop Med Hyg. 2009;81:67–74 .PubMedGoogle Scholar
Figure
Cite This Article1Current affiliation: Ecole Nationale Vétérinaire de Toulouse, Toulouse, France.
Related Links
Table of Contents – Volume 19, Number 5—May 2013
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Amélie Chastagner, INRA, UR346 Epidémiologie Animale, Centre de Recherches de Clermont-Ferrand/Theix, F-63122 Saint Genès Champanelle, France
Top