Volume 20, Number 12—December 2014
Dispatch
Two Outbreaks of Listeria monocytogenes Infection, Northern Spain
Cite This Article
Citation for Media
Abstract
In the province of Gipuzkoa, Spain (≈700,000 inhabitants), 7–12 episodes of human listeriosis were recorded annually during 2009–2012. However, during January 2013–February 2014, 27 episodes were detected, including 11 pregnancy-associated cases. Fifteen cases in 2 epidemiologically unrelated outbreaks were caused by a rare type of Listeria monocytogenes, sequence type 87 serotype 1/2b.
Listeriosis is a bacterial zoonotic infection caused by Listeria monocytogenes. Most human listeriosis episodes are sporadic, but outbreaks affecting a large number of persons can distort the usual annual incidence of infection in a region. Several L. monocytogenes serotypes have been identified, and not all have the same capacity to infect humans; most human cases are caused by serotypes 1/2a, 1/2b, and 4b (1). Although the circulating serotypes have been well defined, little is known about the genetic diversity of L. monocytogenes.
During January 2009–December 2012, the province of Gipuzkoa in Basque Country, northern Spain (≈700,000 inhabitants), recorded 7–12 annual episodes of listeriosis. However, during January 2013–February 2014, a total of 27 human listeriosis episodes were detected in this region. Most of the isolates identified were sequence type (ST) 87 and serotype 1/2b. To date, ST87 represents a rare ST from lineage I that had previously been reported in food (2,3) but had not been shown to cause human infections. We describe 2 epidemiologically unrelated outbreaks of listeriosis caused by ST87 that occurred at the same time in the same region. For 1 of these outbreaks, the causative agent was found in contaminated food.
In Gipuzkoa, 27 human listeriosis episodes were reported during January 2013–February 2014. All cases produced sepsis in the patients, except 1 case that produced diarrheal disease in a 34-year-old parturient woman who had undergone a splenectomy. Eleven episodes (40.7%) occurred in pregnant or parturient women, and 8 of the children of these patients were affected: 5 newborns (4 of them premature infants) became ill, 2 pregnancies ended in miscarriage, and 1 infant was stillborn. (For this study, a pregnancy-associated episode was counted only once, whether the causative strain was isolated from mother, child, or both.) Ten cases (37.0%) occurred among the elderly (>70 years of age) and 6 (22.2%) in adults 45–60 years of age. Of the 6 patients in the 45- to 60-year age group, 3 were apparently healthy and 3 immunocompromised. Three of the 10 elderly patients died.
Human L. monocytogenes isolates were obtained by using routine microbiological procedures. A total of 29 human L. monocytogenes isolates were available for microbiological characterization: 22 from blood, 3 from placental membranes, 2 from cerebrospinal fluid, and 1 each from stool and dermal exudate. Serotypes were established by agglutination (Listeria-O-antisera, Difco; BD Diagnostics, Sparks, MD, USA) and by multiplex-PCR (4). The predominant serotypes identified were 1/2b (n = 17, 58.6%) and 4b (n = 8, 27.6%); 4 isolates were serotype 1/2a (13.8%).
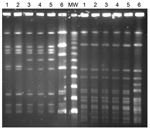
Pulsed-field gel electrophoresis (PFGE) was performed by using the restriction endonucleases SmaI and AscI (5). The STs of L. monocytogenes isolates were determined by using the multilocus sequence typing (MLST) primers and conditions described by the Pasteur Institute (6). MLST showed that 16 of 17 serotype 1/2b isolates we tested were ST87; the remaining serotype 1/2b isolate was ST3. Only 2 human L. monocytogenes ST87 serotype 1/2b isolates, both from Japan, were listed in the Pasteur Institute MLST database (6). Further, PFGE showed 2 large clusters within serotype 1/2b, and epidemiologic research detected 2 main outbreaks. All listeriosis episodes during the study period that were caused by isolate types other than serotype 1/2b and ST87 were sporadic.
In the first case cluster (first outbreak), 5 episodes were detected during August–September 2013; of these, 3 were pregnancy associated. All isolates from 1 woman at 28 weeks’ gestation, 1 parturient woman and her newborn child, and 2 newborn twins (no samples from the mother were available) showed the same PFGE pattern with the restriction enzyme AscI (arbitrarily named as pattern A) and were ST87 (Figure). Another 2 L. monocytogenes isolates from cases apparently not related to this first outbreak, isolated in January 2012 and in April 2013 from a 53-year-old man with meningitis and an 84-year-old woman, respectively, showed the same PFGE pattern.
For this first outbreak, epidemiologic interviews were conducted starting with the cases observed during September 2013. Patients were asked about their alimentary habits, focusing on consumption of raw or semicooked animal or animal derivate products. Although no specific food was identified as the possible source of the outbreak, all but 1 of the patients remembered eating cooked ham bought in the butcher’s department of a certain supermarket chain located in different villages of the province. Listeria spp. in food products were investigated by PCR and culture after selective enrichment (24 Listeria Enrichment Broth; Oxoid, Basingstoke, UK)). In the microbiological investigation (7), 1 of 6 brands of cooked ham studied grew L. monocytogenes at a low level, but the sample strain was not the serotype 1/2b identified in the outbreak. No Listeria spp. were identified in the remaining 5 brands of cooked ham studied.
In the second outbreak, 10 episodes were reported during early November 2013 through late February 2014; patients were 5 elderly persons, 4 previously healthy parturient women, and 1 parturient women who had undergone a splenectomy. No isolates could be obtained from the mother of an infected newborn and from a miscarriage. The 10 isolates available for study (1 per episode) were of the same serotype and sequence type, 1/2b and ST87, as isolates from the first outbreak, but these isolates differed in PFGE AscI pattern (this pattern arbitrarily named pattern B). Isolates from these 2 outbreaks had the same PFGE pattern when the SmaI enzyme was used (Figure).
After the epidemiologic survey for this outbreak, we conducted microbiological analysis of several foods. A foie gras product kept by a patient in his refrigerator yielded a positive culture of L. monocytogenes that had the same phenotype and molecular profile as isolates from the second outbreak. The presence of the outbreak strain was confirmed in high amounts (5.2 × 104 CFU/g) by several other cultures from 3 unopened samples from the same brand of foie gras. After this food source was identified as the source of this second outbreak, all patients from the first outbreak were specifically interviewed again about the consumption of this product, but none remembered having eaten it.
L. monocytogenes infection is serious and has high fatality rates. Among the 35 persons infected in this region of Spain since 2013, a total of 6 deaths have occurred: 3 adults, 2 fetuses (miscarriages), and 1 child (stillbirth). Without rapid case detection and early treatment, the lethality of these infections could have been much higher.
L. monocytogenes infections mainly affect elderly persons, pregnant women, newborns, and immunocompromised adults (8–10). Humans are usually infected after eating contaminated food, although the source of the infection is infrequently detected in sporadic cases. For outbreaks, after the epidemiologic survey, a food is usually implicated as the source of infection, but it is not always possible to obtain microbiological confirmation (11). In this study, the strain (same phenotype and genotype) that caused the second outbreak was obtained from a recently consumed food in the home and in several unopened samples from the same batch of food. However, this source could not be identified as related to the first outbreak.
Pregnant women were frequently infected during these outbreaks; epidemic clones infected 8 pregnant women and their offspring. However, during 2012 and 2013, serotype 4b isolates of different genotypes also infected 5 pregnant women, resulting in 1 miscarriage, 3 premature newborns, and 1 stillborn infant and 1 premature newborn in a twin pregnancy.
In summary, we defined 2 epidemiologically unrelated outbreaks of listeriosis caused by a rare type of L. monocytogenes that occurred at the same time in a small region of Spain. Management of this frequently fatal disease requires careful investigation of the source of infection to stop its spread and prompt treatment of infected persons to prevent severe illness and death.
Dr Pérez-Trallero is a clinical microbiologist and infectious disease consultant. He is head of the Department of Microbiology at Donostia University Hospital and professor of preventive medicine and public health at the Faculty of Medicine at Basque Country University. His research focuses on antimicrobial resistance and epidemiology of transmissible diseases.
Acknowledgment
We thank the team of curators of the Institut Pasteur MLST system (Paris, France) for importing novel alleles, profiles and/or isolates.
References
- Schuchat A, Swaminathan B, Broome C. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–83.PubMedGoogle Scholar
- Chenal-Francisque V, Lopez J, Cantinelli T, Caro V, Tran C, Leclercq A, Worldwide distribution of major clones of Listeria monocytogenes. Emerg Infect Dis. 2011;17:1110–2. DOIPubMedGoogle Scholar
- Wang Y, Zhao A, Zhu R, Lan R, Jin D, Cui Z, Genetic diversity and molecular typing of Listeria monocytogenes in China. BMC Microbiol. 2012;12:119. DOIPubMedGoogle Scholar
- Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J Clin Microbiol. 2004;42:3819–22. DOIPubMedGoogle Scholar
- Standard PulseNet Listeria PFGE–One-Day. (24–28 h). Standardized laboratory protocol for molecular subtyping of Listeria monocytogenes by pulsed field gel electrophoresis (PFGE) [cited 2014 Jun 6]. http://www.pulsenetinternational.org/SiteCollectionDocuments/pfge/5.3_2009_PNetStandProtLMonocytogenes.pdf
- Listeria monocytogenes MLST database [cited 2014 Jun 6]. http://www.pasteur.fr/recherche/genopole/PF8/mlst/Lmono.html
- European Commission (EC). Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. EC; 2007. Official Journal of the European Union [cited 2014 Jun 6]. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:322:0012:0029:EN:PDF
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. EFSA Journal. 2014;12:3547.
- Centers for Diseases and Control and Prevention. Listeria (listeriosis) [cited 2014 Jun 6]. http://www.cdc.gov/listeria/
- Denny J, McLauchlin J. Human Listeria monocytogenes infections in Europe—an opportunity for improved European surveillance. Euro Surveill. 2008;13:pii=8082 [cited 2014 Jun 6]. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=8082
- European Commission Health & Consumer Protection Directorate-General. Opinion of Scientific Committee on Veterinary Measures relating to public health on Listeria monocytogenes (23 September 1999) [cited 2014 Jun 6]. http://ec.europa.eu/food/fs/sc/scv/out25_en.pdf
Figure
Cite This ArticleTable of Contents – Volume 20, Number 12—December 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Emilio Pérez-Trallero, Hospital Donostia—Microbiology, Paseo Dr, Beguiristain s/n San Sebastian Gipuzkoa 20014, Spain
Top