Volume 20, Number 3—March 2014
Letter
Kala-azar and Post–Kala-azar Dermal Leishmaniasis, Assam, India
Cite This Article
Citation for Media
To the Editor: Kala-azar (visceral leishmaniasis) is a fatal disease caused by a protozoan parasite Leishmania donovani and transmitted by the female sandfly, Phlebotomus argentipes. In the state of Assam, India, kala-azar epidemics occurred during 1875–1950 and resulted in thousands of deaths in the districts of Kamrup, Garo Hills, Goalpara, and Nagaon (1,2). The disease gradually disappeared from Assam because of the extensive use of DDT in the national malaria elimination program, and results of later entomologic studies indicated that there were no P. argentipes sandflies in this region after DDT use (3). However, sporadic kala-azar cases appeared again in Assam in 2004 (4), and in 2008, we reported a kala-azar outbreak in Kamrup (5), where kala-azar epidemics had occurred during the 1870s (1).
At bimonthly intervals during 2012, we conducted house-to-house surveys in 4 villages in the district of Kamrup, 845 households and 4,376 persons. Residents are socioeconomically poor and depend on agriculture and nearby brick kiln industries for their livelihood; persons involved in these industries generally keep cattle, and areas of cow manure provide breeding sites for sandflies. Persons reported with fever for >2 weeks, anemia, weight loss, and palpable spleen or liver and who were negative for malaria were tested for kala-azar by using the rK39 diagnostic kit (InBiOS, Seattle, WA, USA). We obtained bone marrow biopsy samples from selected persons who exhibited the symptoms listed above. A total of 162 persons had positive kala-azar results according to rK39 testing during 2008–2012; of these, 44 (27%) were children. Microscopic examination of bone marrow biopsy samples from 5 persons showed L. donovani parasites. We treated kala-azar case-patients with sodium stibogluconate (SSG). During the survey we recorded 4 suspected cases of post–kala-azar dermal leishmaniasis (PKDL).
Case-patient 1, a 16-year-old boy (panel A in Technical Appendix Figure ), was reported positive by rK39 for kala-azar in November 2008. After receiving 30 injections of SSG (20 mg/kg body weight), he became afebrile and his spleen decreased to a nonpalpable size. He gained weight, and hemoglobin improved to reference range. Three years after treatment, hypopigmented macules developed on his face, abdomen, and hands.
Case-patient 2 was an 18-year-old woman (Technical Appendix Figure, panel B). Kala-azar was diagnosed in 2011, and she received 30 injections of SSG. One 1 year after completing treatment, hypopigmented macules developed on her face and hands.
Case-patient 3 was a 16-year-old girl (Technical Appendix Figure, panel C). In 2008, after test results for kala-azar were positive, she received 30 SSG injections and clinically recovered. Macular hypopigmentation developed on her face and body 3.5 years after treatment.
Case-patient 4, a 45-year-old man (Technical Appendix Figure, panel D) was found positive for kala-azar in 2008 and received 17 doses of SSG. He had discontinued treatment because signs and symptoms subsided considerably, and he became afebrile.
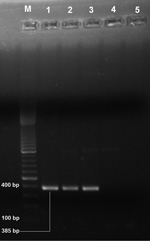
Case-patients 1–4 were clinically examined to exclude other dermal diseases caused by fungi, vitiligo, and leprosy. These persons were also tested, and found to be negative, for tuberculosis, hepatitis C virus, and hepatitis B surface antigen. We obtained punched skin biopsy samples from each case-patient; a pinch of biopsy samples were dab smeared on glass slides for examination for .L donovani parasites, and remaining samples were stored in RNAlater (QIAGEN, Hilden, Germany). We microscopically examined Giemsa-stained slides and found L. donovani parasite in 1 sample. Using QIAamp DNA Mini Kit (QIAGEN), we isolated parasite DNA from the samples and used it for the first round of PCR with primers 5′-AAATCGGCTCCGAGGCGGGAAAC-3′ and 5′-GGTACACTCTATCAGTAGCAC-3′ as described by Salotra et al. (6). Primers encompassing a 385-bp fragment internal to the 592-bp of L. donovani minicircle kinetoplast DNA having sequence 5′-TCGGACGTGTGTGGATATGGC-3′ and 5′-CCGATAATATAGTATCTCCCG-3′ (7) were used for nested PCR. Three samples were positive (Figure). We treated PKDL case-patients with amphotericin B deoxycholate in accordance with World Health Organization guidelines (8), and these patients recovered clinically.
Resurgence of kala-azar in the Kamrup district after a 60-year absence poses new challenges to India’s kala-azar elimination program. Of the 162 kala-azar cases detected, many were in children who had no history of visiting other kala-azar–endemic areas. These findings suggest local transmission of infection and are supported by the presence of the vector sandfly during the 2008 outbreak (5).
In India, PKDL develops in 5%–15% of treated kala-azar case-patients (9); in Sudan, conversion of kala-azar to PKDL is as high as 50% (10). PKDL cases act as reservoirs for kala-azar. Therefore, effective control depends on active surveillance for kala-azar and PKDL and treatment of kala-azar with antileishmanial drugs in accordance with Government of India guidelines (www.nvbdcp.gov.in/Doc/Guidelines-Diagnosis-Treatment-KA.pdf, www.nvbdcp.gov.in/Doc/PKDL-Guidelines-220512.pdf). Ecologic conditions of the areas where kala-azar outbreaks occurred are conducive to sandfly breeding; thus, regular spraying of DDT is needed. Preventive measures to control spread of kala-azar to other areas of Assam would be an effective step for the kala-azar control program.
References
- Rogers L. The epidemic malarial fever of Assam, or kala-azar, successfully eradicated from tea garden lines. BMJ. 1898;2:891–2. DOIPubMedGoogle Scholar
- Price JD, Rogers L. The uniform success of segregation measures in eradicating Kala-azar from Assam tea gardens: it is bearing on the probable mode of infection. BMJ. 1914;1:285–9 and. DOIPubMedGoogle Scholar
- Kaul SM, Sharma RS, Borgohain BK, Das NS, Verghese T. Absence of Phlebotomus argentipes Ann & Brun. (Diptera: Psychodidae) the vector of Indian kala-azar from Kamrup district, Assam. J Commun Dis. 1994;26:68–74 .PubMedGoogle Scholar
- Mathur P, Samantaray JC, Mangraj S. Smoldering focus of kala-azar in Assam. Indian J Med Res. 2004;120:56 .PubMedGoogle Scholar
- Khan AM, Pandey K, Kumar V, Dutta P, Das P, Mahanta J. Sample survey for indigenous cases of kala-azar in Assam by rk39 dipstick test. Indian J Med Res. 2009;129:327–8 .PubMedGoogle Scholar
- Salotra P, Sreenivas G, Pogue GP, Lee N, Nakhasi HL, Ramesh V, Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post–kala-azar dermal leishmaniasis. J Clin Microbiol. 2001;39:849–54 . DOIPubMedGoogle Scholar
- Sreenivas G, Ansari NA, Kataria J, Salotra P. Nested PCR assay for detection of Leishmania donovani in slit aspirates from post–kala-azar dermal leishmaniasis lesions. J Clin Microbiol. 2004;42:1777–8 . DOIPubMedGoogle Scholar
- World Health Organization. Control of leishmaniasis. Technical Report Series 949. Geneva: The Organization; 2010. p. 59–60.
- Salotra P, Singh R. Challenges in the diagnosis of post kala-azar dermal leishmaniasis. Indian J Med Res. 2006;123:295–310 .PubMedGoogle Scholar
- Zijlstra EE, el-Hassan AM. Leishmaniasis in Sudan. Post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95(Suppl 1):S59–76 . DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 20, Number 3—March 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Abdul Mabood Khan, Regional Medical Research Centre, Division of Entomology and Filariasis, Northeastern Region (ICMR) Post Box No. 105, Dibrugarh, Assam 786001, IndiaAbdul Mabood Khan, Regional Medical Research Centre, Division of Entomology and Filariasis, Northeastern Region (ICMR) Post Box No. 105, Dibrugarh, Assam 786001, India
Top