Volume 20, Number 7—July 2014
Letter
Human Exposure to Tickborne Relapsing Fever Spirochete Borrelia miyamotoi, the Netherlands
To the Editor: Borrelia miyamotoi is a relatively novel tickborne relapsing fever spirochete, and is a different species than B. burgdorferi sensu lato, the causative pathogen of Lyme borreliosis (1). B. miyamotoi was first isolated in 1995 from Ixodes persulcatus ticks in Japan, after which it was detected in ticks in North America, Europe, and Russia (1,2). B. miyamotoi infections among humans were first reported in Russia in 2011 (3), and in 2013 in the United States (4). Recently, the first patient infected with B. miyamotoi was reported in the Netherlands (5). Conditions reported to be associated with B. miyamotoi infection were systemic, including malaise and fever, meningoencephalitis, and neurologic symptoms. Because of the nature of these manifestations and because regular diagnostic tests for B. burgdorferi will most probably not detect B. miyamotoi infections (3,5), B. miyamotoi infections may remain undiagnosed. Nevertheless, the relationship between B. miyamotoi infection and illness is not very well established; the case-patients reported, including the patient in the Netherlands, were usually hospitalized, severely ill, and often immunocompromised (3–5). The extent to which B. miyamotoi causes infection and disease in immunocompetent persons is unknown. As a first step to indicate the population at risk for infection, we investigated human exposure to B. miyamotoi in the Netherlands.
To do this, we assessed the B. miyamotoi infection rate of ticks that had bitten humans. Earlier studies included ticks collected through flagging an area (1,2); our study provides specific information about the infection rate of ticks feeding on humans. The ticks were collected from persons who reported their tick bites on the website http://www.tekenradar.nl. After removal of the ticks from the skin, the ticks were submitted to the National Institute of Public Health and the Environment. For 1,040 ticks gathered during April–June 2012, we determined tick species, stage of development, and gender by microscopic examination.
We defined the degree of engorgement in 4 categories from unengorged (score 0) to fully engorged (score 3), as visually determined. To isolate DNA, we boiled the ticks with engorgement scores of 0–1 in ammonium hydroxide (6); for ticks with engorgement scores of 2–3, we used the QIAGEN (Valencia, CA . USA) blood and tissue DNA-extraction kit (7). We used a B. miyamotoi–specific real-time PCR based on the flagellin gene for detection of the bacteria (5). Quantitative PCR-positive tick lysates were tested with a conventional PCR, which amplifies a fragment of glycerophosphodiester phosphodiesterase (glpQ) gene, to confirm the outcome (5). These PCR products were sequenced and were identical to B. miyamotoi sequences filed in GenBank (AB824855). We determined the presence of B. burgdorferi DNA with a duplex quantitative PCR using fragments of the outer membrane protein A gene and the flagellin B gene as targets (7).
All 1,040 ticks were identified as Ixodes ricinus, the most common tick that transmits B. burgdorferi in northern Europe (8). We detected B. miyamotoi DNA in 37 ticks (3.6%) using real-time PCR targeting the flagellin gene, which was confirmed for 32 ticks (3.1%) in the conventional PCR targeting the glpQ gene. (Technical Appendix Table). In 9 of the 37 ticks positive for B. miyamotoi, B. burgdorferi was also detected. Similar to B. burgdorferi, the risk of transmission of B. miyamotoi is likely to become higher if ticks become engorged with blood; 23 of the 37 (62.2%) B. miyamotoi–infected ticks were somewhat engorged (score 1–3) and thus had such an increased risk for transmission. All glpQ sequences of the detected B. miyamotoi isolates were identical to the sequence detected in the sample from the patient reported in the Netherlands this year by Hovius et al. (5). B. burgdorferi DNA was detected in 190 ticks (18.3%) compared with 11.8% detected in a study that included ticks collected through flagging (9).
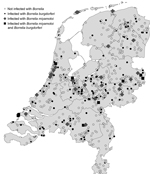
Figure shows that ticks included in the study were submitted from all parts of the country; B. miyamotoi– and B. burgdorferi–positive ticks were found in almost every region. Of the ≈1 million tick bites per year in the Netherlands (10), an estimated 36,000 were by ticks that were infected with B. miyamotoi, and 183,000 were by ticks infected with B. burgdorferi. This substantial human exposure to B. miyamotoi and the reported cases in Russia, the United States, and, recently, the Netherlands (3–5) raises the question to what extent exposure to B. miyamotoi leads to human disease in the general population? These results call for the development of sensitive and specific serologic and molecular tests for B. miyamotoi to identify possible patients, which will lead to a better understanding of the clinical spectrum of B. miyamotoi–induced disease.
Acknowledgment
This study was financed by the Ministry of Health, Welfare and Sport, the Netherlands.
References
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol. 1995;45:804–10 . DOIPubMedGoogle Scholar
- Geller J, Nazarova L, Katargina O, Jarvekulg L, Fomenko N, Golovljova I. Detection and genetic characterization of relapsing fever spirochete Borrelia miyamotoi in Estonian ticks. PLoS ONE. 2012;7:e51914 . DOIPubMedGoogle Scholar
- Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–23 . DOIPubMedGoogle Scholar
- Gugliotta JL, Goethert HK, Berardi VP, Telford SR III. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240–5. DOIPubMedGoogle Scholar
- Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658 . DOIPubMedGoogle Scholar
- Schouls LM, Van De Pol I, Rijpkema SG, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22 .PubMedGoogle Scholar
- Heylen D, Tijsse E, Fonville M, Matthysen E, Sprong H. Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ Microbiol. 2013;15:663–73. DOIPubMedGoogle Scholar
- Heyman P, Cochez C, Hofhuis A, van der Giessen J, Sprong H, Porter SR, A clear and present danger: tick-borne diseases in Europe. Expert Rev Anti Infect Ther. 2010;8:33–50 . DOIPubMedGoogle Scholar
- Coipan EC, Jahfari S, Fonville M, Maassen CB, van der Giessen J, Takken W, Spatiotemporal dynamics of emerging pathogens in questing Ixodes ricinus. Front cell infect microbiol. 2013;3:36.
- Hofhuis A, Harms MG, van der Giessen JWB, Sprong H, Notermans DW, van Pelt W. [Lyme disease in the Netherlands 1994 - 2009]. Infectieziektebulletin 2010;21(3):84–7 [cited 2014 Feb 27] http://www.rivm.nl/Bibliotheek/Algemeen_Actueel/Uitgaven/Infectieziekten_Bulletin/Archief_jaargangen.
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 20, Number 7—July 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
C.C. (Kees) van den Wijngaard, RIVM-Centre for Infectious Disease Control, PO Box 1, 3720 BA Bilthoven, The Netherlands
Top