Volume 20, Number 7—July 2014
Research
Staphylococcus aureus Infections in New Zealand, 2000–2011
Cite This Article
Citation for Media
Abstract
The incidence rate for invasive and noninvasive Staphylococcus aureus infections in New Zealand is among the highest reported in the developed world. Using nationally collated hospital discharge data, we analyzed the epidemiology of serious S. aureus infections in New Zealand during 2000–2011. During this period, incidence of S. aureus skin and soft tissue infections increased significantly while incidence of staphylococcal sepsis and pneumonia remained stable. We observed marked ethnic and sociodemographic inequality across all S. aureus infections; incidence rates for all forms of S. aureus infections were highest among Māori and Pacific Peoples and among patients residing in areas of high socioeconomic deprivation. The increased incidence of S. aureus skin and soft tissue infections, coupled with the demographic disparities, is of considerable concern. Future work should aim to reduce this disturbing national trend.
Despite advances in diagnostics and therapeutics, the clinical and economic burdens of Staphylococcus aureus infections remain a substantial public health problem (1). During the past decade in several parts of the world, most notably in North America, the epidemiology of S. aureus infections has changed dramatically, predominantly because of the epidemic spread of a strain of community-associated methicillin-resistant S. aureus (MRSA) (2,3). Infections caused by community-associated MRSA are most commonly skin and soft tissue infections (SSTIs) and typically occur in patients with no history of exposure to health care facilities (1). In addition, specific sociodemographic associations for community-associated MRSA infection have been described and include younger patient age, specific ethnic groups, and economic deprivation (1,4,5). Although the epidemiology of S. aureus infections has been well studied in North America, comparatively little is known about the trends and patient demographics for S. aureus infections in other geographic settings, particularly in the Southern Hemisphere. Knowledge of the overall prevalence and distribution of S. aureus infections, regardless of methicillin resistance, at a population level is crucial for informing prevention and control strategies.
The incidence of invasive and noninvasive S. aureus infections is reportedly higher In New Zealand than in other developed countries; rates are highest among Māori (indigenous New Zealanders) and Pacific Peoples (6–9). For example, in 1 study, S. aureus bacteremia was 2 times more likely to develop among Māori patients and 4 times more likely to develop among Pacific Peoples than among European patients (7). To date, however, studies describing S. aureus infections in New Zealand have generally been confined to 1 geographic region, to children, or to 1 specific aspect of S. aureus disease such as bloodstream or MRSA infection (4,6–8). Accordingly, we sought to describe the longitudinal trends for S. aureus infection and demographic characteristics of patients across the entire New Zealand population for the 12-year period 2000–2011.
Study Setting
New Zealand is an island nation in the southwestern Pacific Ocean and has ≈4.4 million residents. The population is ethnically diverse, consisting of the following ethnicities: 67% European, 15% Māori, 10% Asian, 7% Pacific Peoples, and 1% other (10). New Zealand has a public health care system; data on all publicly funded hospital admissions are recorded by the New Zealand Ministry of Health in the National Minimum Dataset (NMDS). In addition to basic patient information such as age, sex, and ethnicity, these data include principal and additional hospital discharge diagnoses, which since July 1999 have been coded according to the International Classification of Diseases, Tenth Revision (ICD-10). Our study population included all patients discharged from New Zealand hospitals from January 2000 through December 2011.
Data Collection and Definitions
In New Zealand, a unique identifier (the National Health Index number) is assigned to each person who accesses public health services; this number can be used to extract information from the NMDS about patient hospitalizations. Patients were identified from the NMDS on the basis of S. aureus–associated ICD-10 discharge codes. These ICD-10 codes were A410 (sepsis due to S. aureus), J152 (pneumonia due to staphylococci), and B956 (S. aureus as the cause of diseases classified elsewhere). A case of S. aureus SSTI was defined as infection in a patient who had 1) a principal discharge diagnosis of SSTI (according to an epidemiologic case definition validated in a previous study [11]), 2) an additional discharge diagnosis of B956, and 3) no additional discharge diagnoses containing either A410 or J152. The National Health Index number can also be used to filter out unrelated hospital admissions. We filtered our data to exclude the following groups: overseas visitors, patients on waiting lists, hospital attendees who did not stay overnight, hospital transferees, and patients readmitted to the hospital within 30 days of first admission.
The following information about each patient who was discharged from the hospital for an S. aureus–associated cause was extracted from the NMDS: age, sex, ethnicity, and socioeconomic status (derived from the New Zealand deprivation index [NZDep] (12). The NZDep score is an area-based measure of socioeconomic deprivation derived from New Zealand census data; the score is based on various measures of deprivation, including household income, household ownership, household occupancy, employment and education levels, and access to telecommunications. It is expressed as a score between 1 and 10; a score of 10 represents the most deprived neighborhoods. To determine whether any increasing trends in S. aureus infection were associated with a general increase in all hospital admissions, we obtained information from the NMDS on all patients acutely hospitalized overnight in New Zealand over the study period, applying the same exclusion filters described above.
Statistical Analyses
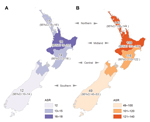
Age-adjusted incidence rates were calculated per 100,000 population and standardized to the age distribution of the 2006 New Zealand census (10). These incidence rates were stratified according to sex, ethnicity, NZDep score, and geographic region. For analysis, we used 4 major ethnic groups: European, Māori, Pacific Peoples, and Asian/other. To determine possible geographic differences in incidence of S. aureus infection across New Zealand, we analyzed 4 broad geographic regions: northern, midland, central, and southern (Figure 1). Population denominator data were obtained from Statistics New Zealand (http://www.stats.govt.nz). A Poisson regression model, with log population data as an offset variable, was used to assess trends over time. The Kruskal-Wallis analysis of variance test was used to determine differences in the geographic incidence of S. aureus infections. Relative risks were calculated with 95% CIs, and all statistical analyses were performed by using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) or STATA version 11.1 (StataCorp, College Station, TX, USA). We considered p<0.05 to be statistically significant.
For the study period, 61,522 S. aureus–associated hospital discharges were identified. The overall averaged 12-year incidence rate for all S. aureus infections was 127 (95% CI 122–133) per 100,000 population per year. The overall incidence rate for S. aureus SSTIs was 108 (95% CI 105–111) per 100,000 population, S. aureus sepsis 14 (95% CI 13–16) cases per 100,000, and staphylococcal pneumonia 5 (95% CI 4–6) cases per 100,000. The incidence rate for sepsis caused by S. aureus and pneumonia caused by staphylococci did not change significantly over the study period; however, the incidence rate for S. aureus SSTIs increased significantly, from 81 (95% CI 78– 84) cases per 100,000 population in 2000 to 140 (95% CI 137–144) cases per 100,000 in 2011 (p<0.001) (Figure 1), which represents an increase of ≈5% each year. In contrast, the rate of acute all-cause hospital discharges in New Zealand fell significantly, from 9,657 (95% CI 9,625–9,689) per 100,000 population in 2000 to 8,701 (95% CI 8,673–8,729) per 100,000 population in 2011 (p<0.001). Consequently, the relative proportion of S. aureus SSTIs to all hospital discharges doubled, from 0.8% in 2000 to 1.6% in 2011.
Incidence of staphylococcal pneumonia did not vary significantly by geographic location (p = 0.8); however, incidence of staphylococcal sepsis (p = 0.02) and SSTIs (p = 0.01) did (Figure 2). In particular, there was a distinct north–south gradient for staphylococcal SSTIs; rates in the northern and central regions were ≈3 times rates in the southern region.
Incidence of S. aureus infections also varied markedly by sociodemographic characteristics (Appendix Table). Staphylococcal infections of all forms were significantly more likely to occur among male than female patients; this difference was most marked for S. aureus sepsis (relative risk [RR] 1.9; 95% CI 1.8–2.0). The incidence rates for sepsis and pneumonia were significantly higher among patients >70 years of age (62 and 24 cases/100,000 population/year, respectively) than among patients of other age groups (Appendix Table). In contrast, the incidence rate for S. aureus SSTIs was highest among those <5 years of age (242 cases/100,000 population/year). The incidence of all disease types was highest among Māori and Pacific Peoples (Appendix Table). In particular, Māori were 3 times more likely and Pacific Peoples almost 5 times more likely than Europeans to have an S. aureus SSTI.
The incidence of S. aureus disease also varied significantly according to socioeconomic deprivation; the incidence rates for sepsis, pneumonia, and SSTI were significantly higher among patients residing in areas of high socioeconomic deprivation. This disparity was most marked for SSTIs; patients residing in areas of high deprivation were almost 4 times more likely to have S. aureus SSTIs than were those residing in areas of low deprivation (RR 3.7, 95% CI 3.6–3.8). An independent association seemed to exist between S. aureus disease and ethnicity after socioeconomic status was adjusted for, such that for each tier of socioeconomic deprivation, all 3 types of S. aureus disease were more common among Māori and Pacific Peoples than among those of European or other ethnicity (Figure 3).
In this study, we analyzed the longitudinal incidence and epidemiology of serious S. aureus disease across the entire New Zealand population during 2000–2011. Incidence of S. aureus SSTI increased dramatically while incidence of S. aureus sepsis and pneumonia remained relatively stable. Our finding of a persistent increase in serious S. aureus SSTIs over the past decade is a substantial public health concern, particularly given the overall decrease in acute overnight hospital admissions in New Zealand.
The factors underlying the increase in such infections are unknown, but risk factors for the development of S. aureus SSTI are multifactorial and probably include household crowding, delayed or inadequate access to health care, and issues associated with household hygiene (13,14). The reasons for the relatively unchanged rate of S. aureus sepsis and staphylococcal pneumonia in New Zealand are unclear; however, recent studies highlight the decreasing incidence of invasive S. aureus infections in other geographic settings, particularly among those patients recently exposed to health care facilities or receiving health care (15,16). Although improvements in infection prevention practices probably contribute to the decrease of invasive infections (17), other possible unexplored factors include changes in host susceptibility to S. aureus infection (e.g., improved management of concurrent conditions such as cardiovascular disease and diabetes) or temporal changes in the virulence profiles or transmissibility of circulating S. aureus strains.
Consistent with the findings of other studies of infectious diseases in New Zealand (13,14), we found notable sociodemographic disparity in the incidence of S. aureus infections; incidence of all S. aureus infections was highest among Māori or Pacific Peoples and among those residing in areas of high socioeconomic deprivation. Even after adjusting for socioeconomic deprivation, we found that the incidence of all S. aureus disease was significantly higher among Māori and Pacific Peoples than among patients of European and other ethnicities; this pattern is seen for infectious diseases generally in New Zealand (14). The underlying reasons for this apparent ethnic disparity in staphylococcal disease are uncertain. Unexplored possibilities include a higher prevalence of S. aureus colonization among Māori or Pacific Peoples or differences in the circulating S. aureus strain types among distinct ethnic groups, as previously described for our setting (4). However, an alternative possibility is that the area-based NZDep score used to record socioeconomic deprivation does not fully represent those facets of poverty that contribute to the development and prevention of serious S. aureus disease. These unmeasured risk factors include aspects of health literacy relating to early management of insect bites and skin infections, availability of household amenities such as hot water, and affordable and timely access to health care. Specific individual-level and household-level studies are required for determination of the relative contribution of such potentially modifiable risk factors.
We also observed significant geographic variation in the incidence of S. aureus SSTI, with a distinct north–south gradient. This finding can probably be explained by the distribution of population groups in New Zealand; the groups most affected by S. aureus SSTI reside predominantly in the North Island (13). However, other possible contributory factors include geographic differences in access to and provision of health care and climate differences; the climate in the upper North Island is relatively warmer and more humid than that in the southern regions.
A limitation of our study was our use of hospital discharge data for case ascertainment. Use of these data meant that we were unable to determine the proportion of cases occurring in the community versus in the hospital setting, although previous studies have demonstrated that most S. aureus infections in New Zealand originate in the community (4,7,8). However, our aim was not to provide detailed information on individual S. aureus infections but rather to provide a broad overview of the trends and demographics of serious S. aureus infections across the entire New Zealand population. In addition, our data represent only those patients whose hospital discharge was associated with S. aureus disease; they do not represent those patients who sought care from a primary care physician or who sought care at a hospital but were not admitted. For example, a recent study of children with SSTIs in 1 New Zealand region found an estimated 14 primary care cases for every 1 hospital admission (18). Furthermore, these data represent only those instances in which an etiologic agent was described and recorded in the discharge diagnoses. It is therefore highly likely that the overall prevalence of staphylococcal disease in our setting is substantially higher than that estimated here.
In summary, our study provides valuable longitudinal data on the prevalence of serious S. aureus disease in the New Zealand population and represents one of the few studies that systematically assessed the epidemiology and demographics of staphylococcal infections across an entire nation. The steady and significant increase in serious S. aureus SSTI coupled with notable sociodemographic disparity in disease incidence is a disturbing national trend. A concerted multimodal public health intervention is urgently required to tackle this problem.
Acknowledgments
This study was supported by internal funding.
Dr Williamson is a clinical microbiologist and a clinical research training fellow of the Health Research Council of New Zealand. Her research interests are the clinical and molecular epidemiology of S. aureus infections and infections caused by antimicrobial drug–resistant pathogens.
References
- David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–87. DOIPubMedGoogle Scholar
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. DOIPubMedGoogle Scholar
- Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–6. DOIPubMedGoogle Scholar
- Williamson DA, Roberts SA, Ritchie SR, Coombs GW, Fraser JD, Heffernan H. Clinical and molecular epidemiology of methicillin-resistant Staphylococcus aureus in New Zealand: rapid emergence of sequence type 5 (ST5)-SCCmec-IV as the dominant community-associated MRSA clone. PLoS ONE. 2013;8:e62020. DOIPubMedGoogle Scholar
- Tong SY, Steer AC, Jenney AW, Carapetis JR. Community-associated methicillin-resistant Staphylococcus aureus skin infections in the tropics. Dermatol Clin. 2011;29:21–32. DOIPubMedGoogle Scholar
- Williamson DA, Ritchie SR, Lennon D, Roberts SA, Stewart J, Thomas MG, Increasing incidence and sociodemographic variation in community-onset Staphylococcus aureus skin and soft tissue infections in New Zealand children. Pediatr Infect Dis J. 2013;32:923–5.PubMedGoogle Scholar
- Hill PC, Birch M, Chambers S, Drinkovic D, Ellis-Pegler RB, Everts R, Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J. 2001;31:97–103. DOIPubMedGoogle Scholar
- Williamson DA, Lim A, Thomas MG, Baker MG, Roberts SA, Fraser JD, Incidence, trends and demographics of Staphylococcus aureus infections in Auckland, New Zealand, 2001–2011. BMC Infect Dis. 2013;13:569. DOIPubMedGoogle Scholar
- El Atrouni WI, Knoll B, Lahr B. EckelaPassow J, Sia I, Baddour LM. Temporal trends in the incidence of Staphylococcus aureus bacteremia in Olmsted County, Minnesota, 1998 to 2005: a population-based study. Clin Infect Dis. 2009;49:e130–8. DOIPubMedGoogle Scholar
- Statistics New Zealand. 2006 census of populations and dwellings. District Health Board area summary tables [cited 2013 Dec 21]. http://www.stats.govt.nz/Census/about-2006-census/district-health-board-area-summary-tables.aspx
- O’Sullivan CE, Baker MG. Proposed epidemiological case definition for serious skin infection in children. J Paediatr Child Health. 2010;46:176–83. DOIPubMedGoogle Scholar
- Salmond C, Crampton P, Sutton F. NZDep91: aNew Zealand index of deprivation. Aust N Z J Public Health. 1998;22:835–7. DOIPubMedGoogle Scholar
- O’Sullivan CE, Baker MG, Zhang J. Increasing hospitalizations for serious skin infections in New Zealand children, 1990–2007. Epidemiol Infect. 2011;139:1794–804. DOIPubMedGoogle Scholar
- Baker MG, Barnard LT, Kvalsvig A, Verrall A, Zhang J, Keall M, Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet. 2012;379:1112–9. DOIPubMedGoogle Scholar
- Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970–8.PubMedGoogle Scholar
- Tracy LA, Furuno JP, Harris AD, Singer M, Langenberg P, Roghmann MC. Staphylococcus aureus infections in US veterans, Maryland, USA, 1999–2008. Emerg Infect Dis. 2011;17:441–8 . DOIPubMedGoogle Scholar
- Roberts SA, Sieczkowski C, Campbell T, Balla G, Keenan A. Implementing and sustaining a hand hygiene culture change programme at Auckland District Health Board. N Z Med J. 2012;125:75–85 .PubMedGoogle Scholar
- O’Sullivan C, Baker MG. Skin infections in children in a New Zealand primary care setting: exploring beneath the tip of the iceberg. N Z Med J. 2012;125:70–9 .PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 20, Number 7—July 2014
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Deborah Williamson, Faculty of Medical and Health Sciences, University of Auckland, Park Rd, Auckland, New Zealand
Top