Volume 20, Number 9—September 2014
Dispatch
Methicillin-Sensitive Staphylococcus aureus CC398 in Intensive Care Unit, France
Cite This Article
Citation for Media
Abstract
During testing for Staphylococcus aureus in an intensive care unit in France in 2011, we found that methicillin-sensitive S. aureus clonal complex 398 was the most frequent clone (29/125, 23.2%). It was isolated from patients (5/89, 5.6%), health care workers (2/63, 3.2%), and environmental sites (15/864,1.7%). Results indicate emergence of this clone in a hospital setting.
Livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) sequence type (ST) 398, which belongs to clonal complex (CC) 398, is an emergent zoonotic agent responsible for massive colonization of livestock and food products and infections in humans worldwide (1). Recently, emergence of animal-independent methicillin-sensitive S. aureus (MSSA) ST398 has been reported in China (2), France (3,4), the Netherlands (5,6), Spain (7), and North America (8–11). MSSA ST398 has been reported in colonized (5,7,8,10,11) and infected (2,5,6,8,10,11) patients. These isolates have been characterized as having staphylococcal protein A (spa) type t571, being sensitive to all antimicrobial drugs except macrolides, and having variable presence of Panton-Valentine leukocidin (2,3,8). In France, an increasing incidence of MSSA ST398 bacteremia has been observed since 2007 (3,4).
During a systematic, molecular, epidemiologic survey of S. aureus in an intensive care unit (ICU) in France, S. aureus CC398 was isolated from patients, health care workers (HCWs), and environmental sites. We conducted a study to describe the spread and characteristics of S. aureus CC398 in this setting.
A prospective molecular epidemiologic study of S. aureus was performed in a 12-bed ICU at the University Hospital in Montpellier, France, during 5 months in 2011. S. aureus nasal carriage was investigated at admission and weekly in 89 patients and monthly in 63 volunteer health care workers (HCWs). Simultaneously, all S. aureus isolates from clinical samples were obtained from the hospital laboratory of bacteriology and clinical data were recorded.
Pneumonia was diagnosed on the basis of clinical, biologic, and radiologic criteria, and a colony count ≥104 CFU/mL in bronchoalveolar fluid culture or ≥107 CFU/mL in sputum cultures. Bronchial colonization was defined as a colony count <107 CFU/mL in sputum cultures in asymptomatic patients.
Random sampling of surfaces was performed monthly in all rooms of the ICU (864 environmental sites). Isolates were characterized by using multilocus sequence typing, double-locus sequence typing (DLST), and accessory gene regulation (agr) typing. Resistance to antimicrobial drugs was detected by using the disk-diffusion method. Virulence genes and ermA, ermC, ermT, and msrA genes were screened for by using PCRs.
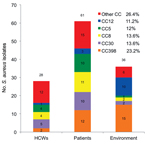
During the survey period, the number of samples obtained ranged from 1 to 32 per patient and from 1 to 3 per HCW. Of these samples, 125 S. aureus isolates (110 MSSA and 15 MRSA) were obtained from 33 patients, 26 HCWs, and 36 environmental sites; these isolates belonged to 28 STs and 12 CCs. Among these 125 isolates, 12 isolates from 5 patients, 2 isolates from 2 HCWs, and 15 isolates from 15 environmental sites belonged to CC398 (Figure 1; Technical Appendix). The 29 strains were MSSA and belonged to ST398 (n = 25) or to a new ST submitted to the MLST Database (http://www.mlst.net/) as ST2658 (n = 4). ST398 and CC398 were the most prevalent genotype and clonal complex identified: 25/125 (20%) and 29/125 (23.2%) isolates, respectively (Figure 2).
The prevalence of MSSA CC398 carriage was 3.2% (2/63) in HCWs and 5.6% (5/89) in patients. The prevalence of MSSA CC398 infection was 2.25% (2/89 patients) (Figure 1). These patients were hospitalized during the same period; nosocomial pneumonia developed after nasal colonization, and was associated with bacteremia in 1 case. Demographic and clinical characteristics were similar in patients colonized or infected with MSSA CC398 or with other genotypes (Table 1). No history of contact with livestock was found for patients and HCWs. The prevalence of MSSA CC398 environmental contamination was 1.7% (15/864 samples). Genotype CC398 was found more frequently in the ICU environment (15/36, 41.7%) than in patients (5/33, 15.2%; χ2 4.7, p = 0.03) and HCWs (2/26, 7.7%; χ2 7.1, p = 0.007) (Technical Appendix).
Molecular typing of CC398 strains and microbiologic results are shown in Table 2. Four strains belonged to the new ST2658, which differed from ST398 by a synonymous mutation (A→G) at position 198 of the pta gene. These 4 strains were isolated from nasal carriage samples (n = 2), bronchial colonization samples (n = 1), and pneumonia testing samples (n = 1) from 2 patients hospitalized at the same time. All MSSA CC398 strains were agr type 1, spa type t571 (determined by using DNAGear software; http://w3.ualg.pt/hshah/DNAGear/), and DLST type 144–186 (DLST spa 186 corresponding to spa type t571). Genes encoding Panton-Valentine leukocidin, toxic shock syndrome toxin 1, and staphylococcal enterotoxin A were not detected. Sensitivity testing of MSSA CC398 isolates showed that all isolates were resistant to erythromycin and had an inducible macrolide–lincosamide–streptogramin B phenotype. In addition, resistance to penicillin and amoxicillin caused by β-lactamase production was observed in 41.4% (12/29) of the strains. Resistance to kanamycin, tobramycin, and gentamicin was observed in 24.1% (7/29) of the strains; all 7 strains were isolated from environmental samples. Analysis of genes encoding antimicrobial drug resistance identified the ermT gene in all the CC398 strains and a variable distribution of ermA and ermC genes.
Identification of MSSA CC398 in HCWs, patients without exposure to livestock, and the environment in an ICU indicates emergence of this clone in a hospital in France. The prevalence of nasal carriage in HCWs and patients was high (≤5.6%) in the context of the ICU, where these persons have frequent contact with each other. The small number of patients colonized or infected with S. aureus CC398 limits statistical comparison of the 2 groups and identification of risk factors for infection.
Despite the monocentric nature and the short period of the study, which limit extrapolation of our results to other settings, our study underlines the capacity of MSSA CC398 to circulate among and between patients, HCWs, and the ICU environment. Slingerland et al. reported prolonged survival of bovine MSSA ST398 strain in the human nose after artificial inoculation, which suggested that competition with human strains might facilitate its spread (12). Identification of ST2658 in 2 patients hospitalized at the same time reinforces the hypothesis of an increased capacity of transmission of this clonal complex between patients.
Person-to-person spread of MSSA ST398 has been reported within community households (8,10) and more recently in a hospital (11) and an urban jail (9), in which a high proportion of detainees sharing a holding tank were colonized with MSSA ST398 (9). These findings contrast with limited transmissibility of livestock-associated MRSA ST398, which is partially explained by molecular signatures of bacterial host adaptation identified only in the MSSA ST398 genome, such as different composition of adhesion genes that result in enhanced adhesion to human skin (10).
All strains were spa type t571, which is the major spa type associated with MSSA ST398 (2,3,5–7). There are other similarities between our strains and strains from China, Spain, Belgium, and the United States. (2,6,7,11), such as agr type 1, the presence of the ermT gene, tetracycline susceptibility, and macrolide–lincosamide–streptogramin B phenotype.
In ICUs, colonized or infected patients constitute the main reservoir of S. aureus (13). The association of MSSA CC398 with the ICU environment suggests that this environment could play a role as a bacterial reservoir as described (14). One hypothesis for such an association is the capacity to form a biofilm, which could be correlated with the S. aureus genetic background (15). Our findings emphasize potential hospital-adapted characteristics of S. aureus CC398, which is supported by others studies (6,11), and indicate that surveillance programs are needed to determine the role of this clonal complex, particularly in the hospital setting.
Dr Brunel is a research student at the Institut de Recherche pour le Développement in Montpellier, France, and a physician in the Department of Infectious Diseases at the University Hospital of Montpellier. Her primary research interests are the epidemiology and control of infectious diseases, in particular, the transmission dynamics of S. aureus in hospital settings.
Acknowledgments
We thank the Institut de Recherche pour le Développement, the Centre National de la Recherche Scientifique, the University of Montpellier 1, and the Laboratory of Bacteriology (Arnaud de Villeneuve Hospital, Montpellier) for their technical support; and HCWs, patients, and patient families in the medical intensive care unit (Gui de Chauliac Hospital, Montpellier) for participating in the study.
This study was supported by a grant from the Association pour l’Assistance et la Réhabilitation à Domicile), Montpellier; and the Institut de Recherche pour le Développement, the Centre National de la Recherche Scientifique, the University of Montpellier 1, and the Laboratory of Bacteriology (Arnaud de Villeneuve Hospital, Montpellier).
References
- Wulf M, Voss A. MRSA in livestock animals: an epidemic waiting to happen? Clin Microbiol Infect. 2008;14:519–21. DOIPubMedGoogle Scholar
- Chen H, Liu Y, Jiang X, Chen M, Wang H. Rapid change of methicillin-resistant Staphylococcus aureus clones in a Chinese tertiary care hospital over a 15-year period. Antimicrob Agents Chemother. 2010;54:1842–7. DOIPubMedGoogle Scholar
- van der Mee-Marquet N, Francois P, Domelier-Valentin AS, Coulomb F, Decreux C, Hombrock-Allet C, Emergence of unusual bloodstream infections associated with pig-borne-like Staphylococcus aureus ST398 in France. Clin Infect Dis. 2011;52:152–3. DOIPubMedGoogle Scholar
- Valentin-Domelier AS, Girard M, Bertrand X, Violette J, Francois P, Donnio PY, Methicillin-susceptible ST398 Staphylococcus aureus responsible for bloodstream infections: an emerging human-adapted subclone? PLoS ONE. 2011;6:e28369. DOIPubMedGoogle Scholar
- van Belkum A, Melles DC, Peeters JK, van Leeuwen WB, van Duijkeren E, Huijsdens XW, Methicillin-resistant and -susceptible Staphylococcus aureus sequence type 398 in pigs and humans. Emerg Infect Dis. 2008;14:479–83. DOIPubMedGoogle Scholar
- Vandendriessche S, Kadlec K, Schwarz S, Denis O. Methicillin-susceptible Staphylococcus aureus ST398-t571 harbouring the macrolide–lincosamide–streptogramin B resistance gene erm(T) in Belgian hospitals. J Antimicrob Chemother. 2011;66:2455–9. DOIPubMedGoogle Scholar
- Lozano C, Gomez-Sanz E, Benito D, Aspiroz C, Zarazaga M, Torres C. Staphylococcus aureus nasal carriage, virulence traits, antibiotic resistance mechanisms, and genetic lineages in healthy humans in Spain, with detection of CC398 and CC97 strains. Int J Med Microbiol. 2011;301:500–5. DOIPubMedGoogle Scholar
- Bhat M, Dumortier C, Taylor BS, Miller M, Vasquez G, Yunen J, Staphylococcus aureus ST398, New York City and Dominican Republic. Emerg Infect Dis. 2009;15:285–7. DOIPubMedGoogle Scholar
- David MZ, Siegel J, Lowy FD, Zychowski D, Taylor A, Lee CJ, Asymptomatic carriage of sequence type 398, spa type t571 methicillin-susceptible Staphylococcus aureus in an urban jail: a newly emerging, transmissible pathogenic strain. J Clin Microbiol. 2013;51:2443–7. DOIPubMedGoogle Scholar
- Uhlemann AC, Porcella SF, Trivedi S, Sullivan SB, Hafer C, Kennedy AD, Identification of a highly transmissible animal-independent Staphylococcus aureus ST398 clone with distinct genomic and cell adhesion properties. MBio. 2012;3:e00027–12 . DOIPubMedGoogle Scholar
- Uhlemann AC, Hafer C, Miko BA, Sowash MG, Sullivan SB, Shu Q, Emergence of Sequence type 398 as a community- and healthcare-associated methicillin-susceptible Staphylococcus aureus in northern Manhattan. Clin Infect Dis. 2013;57:700–3. DOIPubMedGoogle Scholar
- Slingerland BC, Tavakol M, McCarthy AJ, Lindsay JA, Snijders SV, Wagenaar JA, Survival of Staphylococcus aureus ST398 in the human nose after artificial inoculation. PLoS ONE. 2012;7:e48896. DOIPubMedGoogle Scholar
- Cepeda JA, Whitehouse T, Cooper B, Hails J, Jones K, Kwaku F, Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet. 2005;365:295–304. DOIPubMedGoogle Scholar
- Parer S, Lotthé A, Chardon P, Poncet R, Jean-Pierre H, Jumas-Bilak E. An outbreak of heterogeneous glycopeptide-intermediate Staphylococcus aureus related to a device source in an intensive care unit. Infect Control Hosp Epidemiol. 2012;33:167–74. DOIPubMedGoogle Scholar
- Croes S, Deurenberg RH, Boumans ML, Beisser PS, Neef C, Stobberingh EE. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol. 2009;9:229. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 20, Number 9—September 2014
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Anne-Sophie Brunel, Department of Infectious Diseases, University Hospital, 80 Ave Augustin Fliche, Montpellier 34295, France
Top