Volume 21, Number 3—March 2015
Research
Risk Factors for Death from Invasive Pneumococcal Disease, Europe, 2010
Abstract
We studied the possible association between patient age and sex, clinical presentation, Streptococcus pneumoniae serotype, antimicrobial resistance, and death in invasive pneumococcal disease cases reported by 17 European countries during 2010. The study sample comprised 2,921 patients, of whom 56.8% were men and 38.2% were >65 years of age. Meningitis occurred in 18.5% of cases. Death was reported in 264 (9.0%) cases. Older age, meningitis, and nonsusceptibility to penicillin were significantly associated with death. Non–pneumococcal conjugate vaccine (PCV) serotypes among children <5 years of age and 7-valent PCV serotypes among persons 5–64 years of age were associated with increased risk for death; among adults >65 years of age, risk did not differ by serotype. These findings highlight differences in case-fatality rates between serotypes and age; thus, continued epidemiologic surveillance across all ages is crucial to monitor the long-term effects of PCVs.
Streptococcus pneumoniae causes severe invasive disease that results in considerable illness and death. The incidence of invasive pneumococcal disease (IPD) is higher during the early years of life and among elderly persons (1). Geographic and ethnic differences also exist (1,2). Environmental factors (i.e., ambient temperature, humidity, and air pollution) affect IPD incidence (3,4). IPD has also been related to recent respiratory viral infection (4).
The ability of the different S. pneumoniae serotypes to cause disease has been related to serotype-specific characteristics and the molecular size of the capsular polysaccharide and chemical composition, among other factors (5). Therefore, it seems plausible that different serotypes exhibit different virulence and propensity to cause certain clinical presentation (5).
Brueggemann et al. studied the invasive disease potential of different S. pneumoniae serotypes (6). They concluded that so-called “highly invasive” serotypes (including 4, 1, 14, 18C, and 7F), convey a higher risk for invasive disease than do the “low invasive” serotypes (including 3, 15B/C, and 6B), which are more frequently isolated as colonizers (7). Furthermore, serotype distribution varies with patient age, both in disease and in nasopharyngeal colonization (2,8–10). However, evidence exists that pneumococcal invasiveness does not necessarily mean lethality (7). Low invasive serotypes usually account for higher case-fatality rates (CFRs).
The availability of 7-valent, 10-valent, and 13-valent pneumococcal conjugate vaccines (PCV7, PCV10, and PCV13, respectively) and their introduction as part of national immunization schedules have contributed to reducing illnesses and death from IPD (10–12). Nevertheless, the subsequent replacement of vaccine serotypes by nonvaccine serotypes is an accepted and global phenomenon (13,14).
The incidence of drug- and multidrug-resistant S. pneumoniae strains is increasing worldwide (15). Antimicrobial use and abuse is a main driver for the emergence of antimicrobial resistance in respiratory pathogens. Persons who carry (nasopharyngeal colonization), and hence share the potential to transmit resistant pneumococci, also are more susceptible to IPD caused by resistant strains (16).
Monitoring antimicrobial resistance trends and serotype distribution is paramount because this information is essential in helping to determine risk factors and optimizing the appropriate clinical management of cases and public health interventions. We studied the possible association between age, sex, serotype, clinical presentation, antimicrobial resistance, and death among persons reported to have IPD in European countries during 2010.
Data
IPD data derived from passive national surveillance case notification systems were collected during 2010 by 26 European Union (EU)/European Economic Area countries (Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Malta, Netherlands, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden, and United Kingdom); data were submitted to The European Surveillance System. The platform of The European Surveillance System is a metadata-driven system for the collection, validation, cleaning, and analysis of data hosted by the European Centre for Disease Prevention and Control. Surveillance systems differ across Europe, and data were reported with varying levels of completeness. Countries reported only laboratory-confirmed cases based on the EU 2008 case definition.
Study Sample
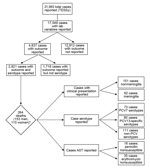
The study sample was the subsample of cases for which information was available about both serotype and outcome (Figure 1). The sample represents data from 17 European countries (Table 1).
Study Variables
An episode of IPD was defined as the isolation of a strain or detection of nucleic acid or antigen of S. pneumoniae from a normally sterile site. Countries reported IPD outcome according to their national surveillance and guidelines. The following age groups were defined for the study: <5 years, 5–64 years, and >65 years. For purpose of this analysis, clinical presentation was recoded as “meningitis” and “nonmeningitis.” Clinical presentation was grouped on the basis of a literature review (5), which suggested that meningitis and nonmeningitis had different degrees of severity and conveyed different rates of death.
Serotypes were grouped into 3 categories: PCV7 serotypes (serotypes in PCV7: 4, 6B, 9V, 14, 18C, 19F, and 23F), PCV13-specific serotypes (serotypes in PCV13 but not in PCV7: 1, 3, 5, 6A, 7F, and 19A), and non-PCV serotypes (serotypes not in any PCV). Results of antimicrobial susceptibility testing to penicillin and erythromycin were reported as “susceptible,” “intermediate,” or “resistant” by the countries according to their national standards and protocols. Therefore, information was not available about the breakpoints and guidelines used for antimicrobial susceptibility testing in each country. For example, in the European Antimicrobial Resistance Surveillance Network report for 2010 (17), 66% of reporting laboratories in Europe used Clinical and Laboratory Standards Institute standards, whereas 29% applied the European Committee on Antimicrobial Susceptibility Testing guidelines.
For this study, we redefined the variable to include just 2 categories: “susceptible” (cases reported as susceptible by the countries) and “nonsusceptible” (intermediate and resistant), both for penicillin and erythromycin. Methods for the characterization of isolates and for antimicrobial susceptibility testing are provided in detail in the 2010 IPD enhanced surveillance report by the European Centre for Disease Prevention and Control (18).
Statistical Analysis
Categorical variables are presented as number of cases and percentages. We used the Pearson χ2 test to compare the proportion of deaths by PCV7, PCV13-specific, and non-PCV serotypes; the proportion of deaths by the defined age groups and by sex; the proportion of deaths by clinical presentation; and the proportion of deaths in antimicrobial-susceptible and -nonsusceptible cases, according to antimicrobial drug type. We used the Fisher exact test to analyze the association between penicillin-susceptible/penicillin-nonsusceptible IDP and outcome for patients <5 years of age and non-PCV serotypes and to assess differences between penicillin-susceptible/penicillin-nonsusceptible cases and outcome for serotype 35B. In addition, we assessed the associations between each serotype and death using a generalized linear model with log-link function. This analysis was performed for the 28 serotypes that accounted for up to 80% of cases with fatal outcomes; each individual serotype was also compared with all the others.
Univariable analysis was performed for the 264 fatal cases to identify factors associated with a fatal outcome. To test the association between age, serotype, clinical presentation, and death, a generalized linear model with robust SEs accounting for the country effect was fitted because data came from different national surveillance systems and vaccination policies and practices differ widely across Europe. We studied the role of variables as potential confounders/modifiers, but only age was statistically significant. Age was an effect modifier of the association between serotype and risk for death, and thus the analysis was stratified by age group.
We also conducted regression analysis. The regression model comprised factors that were significant by univariable analysis and that had previously been hypothesized to affect IPD CFRs.
All p values were 2 tailed, and statistical significance was defined as p<0.05. We conducted statistical analyses by using STATA 12.0 (StataCorp, College Station, TX, USA).
Case Characteristics
In 2010, the European countries reported 21,565 IPD cases. Of these, information was available about laboratory variables for 17,549 cases (Figure 1); outcome was known for 4,637 of these. The study sample comprised 2,921 cases for which information was available about serotype and outcome.
A total of 56.8% of cases (Table 1) occurred in men, and 38.2% of cases were among adults >65 years of age. Children <5 years of age accounted for 19.7% of cases. A total of 264 (9.0%) persons died. Meningitis occurred in 18.5% of cases. PCV13-specific serotypes (1, 3, 5, 6A, 7F, 19A) accounted for 42.7% of cases. Nonsusceptibility (intermediate + resistant) to penicillin was reported in 122 (5.9%) of 2,071 cases; nonsusceptibility to erythromycin was reported in 486 (23.6%) of 2,059 cases (Table 1).
PCV13-specific serotypes caused 57.7% (p<0.001) of cases among children <5 years of age (Figure 2). Non-PCV serotypes accounted for 48.0% of cases among adults >65 years of age. Meningitis cases were predominantly caused by non-PCV serotypes (41.4%, p<0.001) (Figure 2). Nonsusceptibility to penicillin was highest among PCV7 serotypes (64.8%, p<0.001) (Figure 2).
The Pearson χ2 analysis (Table 2) demonstrated a lack of statistical association between sex and death (p = 0.631). The CFR was highest for adults >65 years of age (13.7%, p<0.001); 2.3% of children <5 years of age died.
Clinical presentation was associated with death. The CFR for persons with meningitis was 15.9% compared with 8.8% for those without meningitis (p<0.001).
Death was also associated with nonsusceptibility to penicillin. Death occurred in 13.1% of cases in which S. pneumoniae was not susceptible to penicillin (p = 0.010) (Table 2). Nonsusceptibility to erythromycin was not significantly associated with death (p = 0.837).
We determined the association between individual serotype and death (Table 3). Serotype 35B (relative risk [RR] 4.98, 95% CI 2.49–9.95), serotype 4 (RR 2.03, 95% CI 1.04–3.95), and serotype 11A (RR 1.97, 95% CI 1.33–2.94) were most associated with death. Serotype 3 (RR 1.39, 95% CI 0.88–2.21) accounted for the highest number and the highest percentage (13.3%) of serotype-specific deaths, but the association with death was not statistically significant (p = 0.161). In contrast, for serotype 1 (RR 0.25, 95% CI 0.13–0.48) and serotype 5 (RR 0.15, 95% CI 0.09–0.26), there was no association with death. Subanalysis of the association between susceptibility to penicillin and outcome for serotype 35B found no significant differences in risk for death between susceptible and nonsusceptible cases.
Risk Factors for IPD-Associated Death
Univariable analysis showed differences between nonfatal and fatal cases (Table 4). Persons 5–64 years of age (RR 3.55, 95% CI 1.66–7.61) and >65 years of age (RR 4.79, 95% CI 3.08–11.76) had a higher risk for death than did children <5 years of age. In the univariable analysis, meningitis (RR 1.81, 95% CI 1.25–2.61, p = 0.002) was significantly associated with death. PCV7 serotypes were also significantly associated with death (RR 2.18, 95% CI 1.06–4.48, p = 0.034). Conversely, non-PCV serotypes were not associated with death (RR 1.47, 95% CI 0.94–2.28).
Nonsusceptibility to penicillin was associated with increased risk for death (RR 1.91, 95% CI 1.16–3.13). Nonsusceptibility to erythromycin was not significantly associated with death (RR 1.04, 95% CI 0.84–1.29).
Our comparison of susceptibility to penicillin and outcome for clinical presentation showed that the association with the outcome remained statistically significant only for meningitis cases (RR 1.82, 95% CI 1.27–2.62, p = 0.001). These factors were not associated with nonmeningitis cases (RR 1.31, 95% CI 0.28–6.01).
Age was an effect modifier. In the stratified analysis, we found that among children <5 years of age, risk for death from non-PCV serotypes increased (RR 3.68, 95% CI 1.27–10.69) (Table 5), whereas among persons 5–64 years of age, PCV7 serotypes conveyed the highest risk for death (RR 2.68, 95% CI 1.37–5.23). Among adults >65 years of age, risk for death among the serotypes did not differ significantly.
We analyzed the association between susceptibility to penicillin and outcome for non-PCV serotypes. Children <5 years of age showed no differences between susceptible and nonsusceptible cases.
Our analysis of IPD surveillance data from Europe in 2010 unveiled a significant association between death and older age, meningitis, serotypes contained in PCV7, and nonsusceptibility to penicillin. As have many other studies, we found an association between increased age and death (19–22). The risk was higher for adults >65 years of age (RR 4.79, 95% CI 3.08–11.76) than for persons 5–64 years of age (RR 3.55, 95% CI 1.66–7.61). However, the lack of information about patients’ clinical characteristics impedes accurate assessments of these differences.
Elderly persons have been postulated to have an increased susceptibility to—in addition to co-ocurring conditions—pneumococcal disease because of reduced splenic function (23), age-related changes in respiratory tract, immunosenescence, and cellular senescence related to age-associated inflammation (23). The higher incidence and death rates for IPD in this age group is remarkable and highlights the need to direct vaccination toward the elderly. These findings may present an opportune moment to revisit adult vaccination recommendations and programs in European countries (24).
We did not find sex to be significantly associated with death. However, other studies have shown association either with men (25) or women (23,26).
In our study, presence of meningitis was significantly associated with death. Harboe et al. obtained similar results in a large population-based cohort study (25). In Denmark, another study concluded that patients with pneumococcal meningitis had increased death rates, but these rates derived from severe underlying conditions (27). CFRs for pneumococcal meningitis are usually higher than for nonmeningitis (28). More recently, Ladhani et al. found that the CFR was higher for children with meningitis in England and Wales (29). This study showed that infecting serotype was not associated with death (29), whereas meningitis and co-occurring conditions were significantly associated with death. In our analysis, meningitis was predominantly caused by non-PCV serotypes; this finding could be an effect of PCV introduction, as observed in other studies (30). Another analysis of susceptibility to penicillin by clinical presentation showed a higher risk for death among persons with nonsusceptible IPD than for those with susceptible IPD who had meningitis. Therefore, in the absence of information about clinical management of cases and existing co-occurring conditions, the association between meningitis and nonsusceptibility to penicillin might be an explanation.
Capsular differences between serotypes affect clinical presentation and outcome (10,31,32). These differences are in accordance with our study, which found PCV7 serotypes were associated with death in the univariable analysis. Among children <5 years of age, PCV13-specific serotypes were most frequently identified, compared with PCV7 and non-PCV serotypes, as defined in our study. In 2010, PCV13 was already licensed, and many European countries began moving from PCV7 to the higher-valent vaccine, although with different schemes, policies, and dates of introduction. Nevertheless, these changes are unlikely to have affected our study findings because we analyzed data from 2010.
After stratification, the highest risk for death among children <5 years of age corresponded to non-PCV serotypes. This finding could be attributed to serotype replacement after pneumococcal vaccination (29,30). Our analysis found no differences between penicillin-susceptible and -nonsusceptible cases among children <5 years of age and non-PCV serotypes subgroup with respect to death. However, the overall percentage of meningitis cases was high (18.5% of the study sample), and meningitis was predominantly caused by non-PCV serotypes (p<0.001) (Figure 2). Hence, vaccines with enhanced serotype coverage (higher valency) might be needed to prevent IPD in this age group in the near future.
Among persons 5–64 years of age, the risk for death was highest for PCV7 serotypes, which were predominantly nonsusceptible to penicillin (p<0.001) (Figure 2). Reductions in IPD caused by PCV7 serotypes in non–vaccine-eligible age groups in countries with universal use of PCV7 might indicate the indirect effect of PCV7 (33). However, because vaccine policies differed among European countries at the time of the study, this indirect effect might not be reflected in the pooled data (Table 6).
Serotypes 1, 5, and 7F have been described as having high potential for invasiveness (these serotypes are carried for a short time) but are associated with milder disease and lower CFRs (7,9,19,34). As in those studies, we found that serotypes 1 and 5 caused IPD but were not associated with death.
Serotype 35B has been reported as nonsusceptible to penicillin (35). The subanalysis on susceptibility to penicillin for serotype 35B showed that penicillin nonsusceptibility did not affect the risk for death for serotype 35B. Nevertheless, the increased risk for death of non-PCV serotypes 11A and 35B merits further monitoring.
We found penicillin nonsusceptibility to be significantly associated with death, as described by others (20,36). Nevertheless, in other large studies, this association was not found (21,26,34,37), and the effect of multidrug-resistant strains remains to be determined. Conversely, we found that erythromycin nonsusceptibility did not significantly affect death, as described by Song et al. (37) and Martens et al. (20). A plausible explanation might be the additional benefits of macrolides (i.e., their immunomodulatory/antiinflamatory properties), which might be important when these drugs are used in combination with other therapeutic agents (38).
Antimicrobial resistance to S. pneumoniae is increasing in many countries in Europe (17), and the prudent use of antibacterial drugs, apart from immunization, is pivotal in preventing and controlling IPD. Furthermore, these findings underpin the importance of antimicrobial susceptibility testing to assist with the clinical management of cases and to provide data on prevalence of antimicrobial resistance.
The major strength of our study is its large sample size; data were from national surveillance systems across Europe (i.e., we analyzed IPD individual-level data from populations in a large geographic area). In 2010, European IPD surveillance collected data corresponding to ≈82% of the total population of EU/European Economic Area countries. This enhanced surveillance for IPD data pooled together at supranational level enables comparisons with other parts of the world.
Despite its strengths, our study has some limitations. Surveillance of IPD varies markedly in Europe, including differences in laboratory methods to confirm cases, reporting, and medical practices. Therefore, a certain degree of underdiagnosis and underreporting are likely to exist in this dataset. Moreover, surveillance systems for IPD differ in sensitivity, representativeness, and specificity across Europe; these variations might have influenced the results because some countries were major contributors (Table 7) and ascertainment bias might have also occurred. Information about co-occurring conditions or clinical management of cases that might have affected outcome was also missing. European countries introduced pneumococcal vaccination at different times and with different policies, which might have affected the serotype distribution throughout Europe. Furthermore, the incomplete information about the vaccination status of cases makes difficult the interpretation of results. These limitations emphasize the need for continued and improved surveillance of IPD throughout Europe.
In conclusion, we found that older age, meningitis, non-PCV serotypes among children <5 years of age and PCV7 serotypes among persons 5–64 years of age, and penicillin nonsusceptibility were risk factors for death from IPD in Europe. The stratified analysis highlighted differences in risk for death according to S. pneumoniae serotype and age group. This knowledge may assist in decision making when implementing vaccination strategies as new immunization strategies are needed to tackle the considerable IPD and associated death in adults (39) and in designing new extended valency vaccines or protein-based pneumococcal vaccines that may confer serotype-independent immunity (40).
Dr. Navarro-Torné is an expert in vaccine preventable diseases at the European Centre for Disease Prevention and Control. Her primary research interest is surveillance of vaccine preventable diseases, particularly on IPD and pertussis.
Acknowledgment
Members of the Invasive Pneumococcal Disease Study Group who contributed data this article: Martine Sabbe, Antoaneta Detcheva, Teodora Georgieva, Despo Pieridou Bagatzouni,Pavla Křížová, Palle Valentiner-Branth, Asunción Fenoll, Anni Virolainen-Julkunen,Theano Georgakopoulou, Georgina Tzanakaki, Márta Melles, Imelda Vickers, Suzanne Cotter, Hilary Humphreys, Karl G. Kristinsson, Maria Grazia Caporali, Fortunato Paolo D’Ancona, Stefania Iannazzo, Annalisa Pantosti, Jelena Galajeva, Paul Caruana, Jackie Maistre Melillo, Tanya Melillo Fenech, Hester De Melker, Mirjam Knol, Arie Van Der Ende, Martin Steinbakk, Didrik F Vestrheim,Waleria Hryniewicz, Alicja Kuch, Iwona Paradowska-Stankiewicz, Anna Skoczynska, Marina Pana, Birgitta Henriques-Normak, Alenka Kraigher, Maja Sočan, Helena Hupkova, Eisin MacDonald, Mary P.E. Slack, Pauline A. Waight.
References
- Lynch JP, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance and impact of vaccines. Curr Opin Pulm Med. 2010;16:217–25 .PubMedGoogle Scholar
- Scott JA, Hall AJ, Dagan R, Dixon JMS, Eykyn SJ, Fenoll A, Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex and geography in 7000 episodes of invasive disease. Clin Infect Dis. 1996;22:973–81. DOIPubMedGoogle Scholar
- Weinberger DM, Grant LR, Steiner CA, Weatherholtz R, Santosham M, Viboud C, Seasonal drivers of pneumococcal disease incidence: impact of bacterial carriage and viral activity. Clin Infect Dis. 2014;58:188–94. DOIPubMedGoogle Scholar
- Launes C, Fernandez de Sevilla M, Selva L, Garcia Garcia JJ, Pallares R, Muñoz-Almagro C. Viral co-infection in children less than five years old with invasive pneumococcal disease. Pediatr Infect Dis J. 2012;31:650–3. DOIPubMedGoogle Scholar
- Weinberger DM, Harboe ZB, Sanders EAM, Ndiritu M, Klugman KP, Rückinger S, Risk of death from pneumococcal pneumonia is a stable serotype-associated property: a meta-analysis. Clin Infect Dis. 2010;51:692–9. DOIPubMedGoogle Scholar
- Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. 2003;187:1424–32. DOIPubMedGoogle Scholar
- Pletz MW, Welte T, Klugman KP. The paradox in pneumococcal serotypes: highly invasive does not mean highly lethal. Eur Respir J. 2010;36:712–3. DOIPubMedGoogle Scholar
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:e1000348. DOIPubMedGoogle Scholar
- Jansen AGSC, Rodenburg GD, van der Ende A, van Alphen L, Veenhoven RH, Spanjaard L, Invasive pneumococcal disease among adults: association among serotypes, disease characteristics, and outcome. Clin Infect Dis. 2009;49:e23–9. DOIPubMedGoogle Scholar
- Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5:83–93. DOIPubMedGoogle Scholar
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine. 2011;29:9127–31. DOIPubMedGoogle Scholar
- Palmu AA, Jokinen J, Nieminen H, Borys D, Nieminen H, Ruokokoski E, Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomized trial. Lancet. 2013;381:214–22. DOIPubMedGoogle Scholar
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. DOIPubMedGoogle Scholar
- Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis. 2012;12:207. DOIPubMedGoogle Scholar
- Reinert RR. The antimicrobial resistance profile of Streptococcus pneumoniae. Clin Microbiol Infect. 2009;15(Suppl 3):7–11. DOIPubMedGoogle Scholar
- Dagan R, Barkai G, Leibovitz E, Dreifuss E, Greenerg D. Will reduction of antibiotic use reduce antibiotic resistance? The pneumococcus paradigm. Pediatr Infect Dis J. 2006;25:981–6. DOIPubMedGoogle Scholar
- European Antimicrobial Resistance Surveillance Network (EARS-Net). Antimicrobial resistance interactive database (EARS-Net) [cited 2014 Mar 2]. http://www.ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database/Pages/database.aspx
- European Centre for Disease Prevention and Control. Surveillance of invasive pneumococcal disease in Europe, 2010 [cited 2014 Mar 3]. http://www.ecdc.europa.eu/en/publications/publications/invasive-pneumoccocal-disease-surveillance-2010.pdf
- Luján M, Gallego M, Belmont Y, Fontanals D, Vallés J, Lisboa T, Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. Eur Respir J. 2010;36:1073–9. DOIPubMedGoogle Scholar
- Martens P, Worm SW, Lundgren B, Konradsen HB, Benfield T. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infect Dis. 2004;4:21. DOIPubMedGoogle Scholar
- Yu VL, Chiou CCC, Feldman C, Ortqvist A, Rello J, Morris AJ, An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37:230–7. DOIPubMedGoogle Scholar
- Regev-Yochay G, Rahav G, Riesenberg K, Wiener-Well Y, Strahilevitz J, Stein M, Initial effects of the national PCV7 childhood immunization program on adult invasive pneumococcal disease. PLoS ONE. 2014;9:e88406. DOIPubMedGoogle Scholar
- Simell B, Lahdenkari M, Reunanen A, Käyhty H, Väkeväinen M. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin Vaccine Immunol. 2008;15:1391–7. DOIPubMedGoogle Scholar
- European Centre for Disease Prevention and Control. Vaccine schedule. Recommended immunisations for pneumococcal disease [cited 2014 Apr 7]. http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx
- Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6:e1000081. DOIPubMedGoogle Scholar
- Vallès X, Marcos A, Pinart M, Piñer R, Marco F, Mensa JM, Hospitalized community-acquired pneumonia due to Streptococcus pneumoniae. Has resistance to antibiotics decreased? Chest. 2006;130:800–6. DOIPubMedGoogle Scholar
- Roed C, Engsig FN, Omland LH, Skinhoj P, Obel N. Long-term mortality in patients diagnosed with pneumococcal meningitis: a Danish nationwide cohort. Am J Epidemiol. 2010;172:309–17. DOIPubMedGoogle Scholar
- Rückinger S, von Kries R, Siedler A, van der Linden M. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr Infect Dis J. 2009;28:118–22. DOIPubMedGoogle Scholar
- Ladhani SN, Slack MPE, Andrews NJ, Waight PA, Borrow R, Miller E. Invasive pneumococcal disease after routine pneumococcal conjugate vaccination in children, England and Wales. Emerg Infect Dis. 2013;19:61–8. DOIPubMedGoogle Scholar
- Pichon B, Ladhani SN, Slack MPE, Segonds-Pichon A, Andrews NJ, Waight PA, Changes in molecular epidemiology of Streptococcus pneumoniae causing meningitis following introduction of pneumococcal conjugate vaccination in England and Wales. J Clin Microbiol. 2013;51:820–7. DOIPubMedGoogle Scholar
- Li Y, Weinberger DM, Thompson CM, Trzciński K, Lipsitch M. Surface charge of Streptococcus pneumoniae predicts serotype distribution. Infect Immun. 2013;81:4519–24. DOIPubMedGoogle Scholar
- van Hoek AJ, Andrews N, Waight PA, George R, Miller E. Effect of serotype on focus and mortality of invasive pneumococcal disease: coverage of different vaccines and insight into non-vaccine serotypes. PLoS ONE. 2012;7:e39150. DOIPubMedGoogle Scholar
- Myint TTH, Madhava H, Balmer P, Christopoulou D, Attal S, Menegas D, The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Adv Ther. 2013;30:127–51. DOIPubMedGoogle Scholar
- Sjöström K, Spindler C, Örtqvist A, Kalin M, Sandgren A, Kühlmann-Berenzon S, Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis. 2006;42:451–9. DOIPubMedGoogle Scholar
- Fenoll A, Aguilar L, Giménez MJ, Vicioso MD, Robledo O, Granizo JJ, Susceptibility of recently collected Spanish pneumococci nonsusceptible to oral penicillin from serotypes not included in the 7-valent conjugate vaccine. Antimicrob Agents Chemother. 2010;54:2696–8. DOIPubMedGoogle Scholar
- Gouveia EL, Reis JN, Flannery B, Cordeiro SM, Lima JBT, Pinheiro RM, Clinical outcome of pneumococcal meningitis during the emergence of penicillin-resistant Streptococcus pneumoniae: an observational study. BMC Infect Dis. 2011;11:323. DOIPubMedGoogle Scholar
- Song JS, Choe PG, Song KH, Park WB, Park SW, Kim HB, Risk factors for 30-day mortality in adult patients with pneumococcal bacteraemia, and the impact of antimicrobial resistance on clinical outcomes. Epidemiol Infect. 2012;140:1267–76. DOIPubMedGoogle Scholar
- Feldman C, Anderson RA. Bacteremic pneumococcal pneumonia: current therapeutic options. Drugs. 2011;71:131–53. DOIPubMedGoogle Scholar
- Vila-Corcoles A, Ochoa-Gondar O. Preventing pneumococcal disease in the elderly. Recent advances in vaccines and implications for clinical practice. Drugs Aging. 2013;30:263–76. DOIPubMedGoogle Scholar
- Miyaji EN, Sarno Oliveira ML, Carvalho E, Lee Ho P. Serotype-independent pneumococcal vaccines. Cell Mol Life Sci. 2013;70:3303–26 and. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1Members of the Invasive Pneumococcal Disease Study Group who contributed data are listed at the end of this article.
Table of Contents – Volume 21, Number 3—March 2015
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Adoración Navarro-Torné, European Centre for Disease Prevention and Control—Surveillance and Response Support Unit, Tomtebodavagen 11a 17183 Stockholm 17183, Sweden
Top