Volume 21, Number 4—April 2015
Dispatch
La Crosse Virus in Aedes japonicus japonicus Mosquitoes in the Appalachian Region, United States
Abstract
La Crosse virus (LACV), a leading cause of arboviral encephalitis in children in the United States, is emerging in Appalachia. For local arboviral surveillance, mosquitoes were tested. LACV RNA was detected and isolated from Aedes japonicus mosquitoes. These invasive mosquitoes may significantly affect LACV range expansion and dynamics.
La Crosse virus (LACV; family Bunyaviridae, genus Orthobunyavirus), in the California serogroup, is the major cause of arboviral encephalitis among children in the United States (1). Since its 1963 discovery in Wisconsin, LACV has been identified in 30 other US states (2). These include states within the Appalachian Mountain region (West Virginia, Virginia, Ohio, Tennessee, and North Carolina), which is an emerging focus of LACV (3).
The primary vectors of LACV, Aedes triseriatus mosquitoes, are present in southwestern Virginia and West Virginia, but 2 invasive congeners—Ae. albopictus and Ae. japonicus—have recently emerged (3). Both species have been shown to be competent experimental LACV vectors (4,5). Although LACV has been isolated from Ae. albopictus mosquitoes (6), previously it had only been detected in the Asian bush mosquito (Ae. japonicus japonicus) in Tennessee (7). Ae. japonicus mosquitoes are mammalophilic container breeders that co-occur with the primary LACV vector (Ae. triseriatus mosquitoes). Known to feed on humans (8), Ae. japonicus mosquitoes are found in woodlands (where this “rural encephalitis” virus is endemic) and urban areas (9).
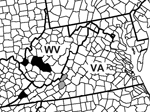
To ascertain the public health risk that Ae. japonicus mosquito vectors represent for LACV transmission, we examined mosquitoes from West Virginia and Virginia for presence of this arbovirus. We report 2 independent isolations of LACV from adult Ae. japonicus mosquitoes in southwestern Virginia and 7 field detections of LACV RNA from adults (Virginia and West Virginia) and adults reared from eggs (Virginia). Our findings suggest a potential role of this invasive vector in the ecology of LACV in Appalachia (Figure 1).
In 2005, mosquito eggs were collected weekly in Wise County, Virginia, by using ovitraps. The resultant larvae were reared to adults in a Biosafety Level 2 insectary at 24°C, 75% relative humidity, and a photoperiod of 16 hours of light and 8 hours of dark. In 2008 and 2009, adult mosquitoes were collected weekly from infusion-baited gravid traps in Montgomery and Craig Counties, Virginia. Mosquitoes were identified to the species level according to morphology and grouped in pools of <50 individuals according to species, collection location, and collection date. Adults were stored at −80°C until testing. Reverse transcription PCR (RT-PCR) was used for LACV detection in mosquitoes collected in 2005 and 2008. In 2009, mosquito pools were homogenized by using previously described methods for LACV isolation (6). Homogenate supernatant (150 μL) was inoculated onto Vero cells, incubated at 37°C, and monitored daily for cytopathic effect. Isolates that showed marked cytopathic effect were harvested and submitted to the Centers for Disease Control and Prevention (CDC) in Fort Collins, Colorado, USA, and the Wadsworth Center in Slingerlands, New York, USA, for quantitative RT-PCR (qRT-PCR).
In 2013, mosquito surveillance was conducted as part of the West Virginia Department of Health and Human Resources Mosquito Surveillance Program. Gravid traps, carbon dioxide–emitting light traps, and BG Sentinel (Biogents AG, Regensburg, Germany) traps baited with octenol lures were used to collect adult mosquitoes weekly from counties with high (Nicholas, Fayette, Raleigh) and low (Kanawha, Jackson, Wood) incidence of LACV among humans as defined (10) and on a somewhat regular basis in additional counties. Specimens were pooled by species, county, and collection date for qRT-PCR at the West Virginia Office of Laboratory Services, by use of previously described methods (11).
LACV RNA was detected in a pool of Ae. japonicus mosquitoes collected as eggs in August 2005 from Wise County, Virginia. LACV was also detected in a pool of Ae. japonicus mosquitoes from Montgomery County, Virginia, in July 2008. LACV RNA was detected in 5 separate pools of Ae. japonicus mosquitoes collected in West Virginia in 2013, representing 3 counties over a 4-month period. Of 3,529 Ae. japonicus mosquitoes collected from Montgomery County in 2009, we isolated LACV from 1 pool (n = 3). In that same year, of 796 Ae. japonicus mosquitoes from Craig County tested, LACV was isolated from 1 pool (n = 50) (Table). These isolations were verified by qRT-PCR at CDC (Montgomery County isolate) and the Wadsworth Center (Craig County isolate).
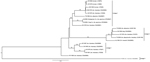
Nucleotide sequencing and a BLAST (http://blast.ncbi.nlm.nih.gov//Blast.cgi) query were performed on the amplified cDNA from both isolates. The LACV medium segment was used to infer phylogeny (Figure 2). Coding sequences were aligned in Mesquite version 2.75 with Opalescent version 2.10 (12,13). Phylogenetic trees for the polyprotein genes were estimated by using a maximum likelihood–based method and assuming a general time-reversible (GTR) model with gamma-distributed rate heterogeneity of nucleotide substitution GTR + Γ in RAxML version 8.0.0 (14). Support values for each clade were generated in RAxML by using 1,000 rapid bootstrap replicates. The Virginia 2009 isolates were within the previously described lineage I, which contains the reference LACV strain isolated from the brain tissue of a child in Wisconsin (National Center for Biotechnology Information accession no. U18979) (15). The 2 isolates from mosquitoes collected in Virginia in 2009 differed by only 1 bp.
The isolation of LACV from field-collected Ae. japonicus mosquitoes, and particularly from mosquitoes collected as eggs, is highly significant because of the pervasiveness of this species in the United States. The large number of LACV detections in this invasive species highlights the need for LACV mosquito surveillance and control efforts to include Ae. japonicus in addition to Ae. triseriatus mosquito populations. Most (3/5) detections of LACV in West Virginia were in mosquitoes from Fayette County, where incidence of LACV among humans is high, suggesting that Ae. japonicus mosquitoes may play a major role in transmission of LACV to humans. Our detection of LACV in Ae. japonicus mosquitoes from field-collected eggs in 2005 and the ability of LACV to be transmitted transovarially in Ae. triseriatus mosquitoes suggests that future research should examine the possibility of vertical transmission of LACV in invasive Ae. japonicus mosquitoes. In states east of the Mississippi River, where Ae. japonicus mosquitoes (9) and LACV (2) co-exist, this mosquito may play a major role in the maintenance, transmission, and range expansion of LACV.
Dr. Harris is a wildlife veterinarian and disease ecologist. Her primary research interest is understanding how anthropogenic environmental changes affect disease dynamics, especially zoonotic and vectorborne diseases.
Acknowledgments
We thank Amy Lambert for conducting RT-PCR and sequencing of the 2009 isolate from Montgomery County, Virginia; Dee Petit and Andrew Luna for technical assistance; and Nate Lambert, Allen Patton, Bonnie Fairbanks, Jennifer Miller, and Laila Kirkpatrick for field and laboratory assistance. In West Virginia, Christi Clark, Chris Boner, and Lindsay Kuncher provided laboratory assistance, and field surveillance was conducted by Kristin Alexander, Stephen Catlett, Hannah Cavender, Robert Deneer, Jennifer Beamer Hutson, Mickey King-Fowler, Dustin Mills, Daniel Payne, and Courtney Stamm. We thank the Wadsworth Center sequencing core for sequencing 1 isolate.
Mosquito surveillance in West Virginia was supported in part by a CDC Epidemiology and Laboratory Capacity for Infectious Diseases grant. Contributions of M.C.H. were supported in part by a National Institutes of Health Ruth L. Kirschstein National Research Service Award for Individual Predoctoral Fellows (no. 1F31AI080160-01A1).
References
- McJunkin JE, de los Reyes EC, Irazuzta JE, Caceres MJ, Khan RR, Minnich LL, La Crosse encephalitis in children. N Engl J Med. 2001;344:801–7. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Confirmed and probable California serogroup virus neuroinvasive disease cases, human, United States, 1964–2010, by state (as of 5/9/2011) [cited 2014 Dec 16]. http://www.cdc.gov/lac/resources/CAL_LAC.pdf
- Grim DC, Jackson BT, Paulson SL. Abundance and bionomics of Ochlerotatus j. japonicus in two counties in southwestern Virginia. J Am Mosq Control Assoc. 2007;23:259–63 . DOIPubMedGoogle Scholar
- Grimstad PR, Kobayashi JF, Zhang M, Craig GB. Recently introduced Aedes albopictus in the United States–potential vector of La Crosse virus (Bunyaviridae, California serogroup). J Am Mosq Control Assoc. 1989;5:422–7 .PubMedGoogle Scholar
- Sardelis MR, Turell MJ, Andre ARG. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae). J Med Entomol. 2002;39:635–9. DOIPubMedGoogle Scholar
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001;7:807–11.PubMedGoogle Scholar
- Westby K, Fritzen C, Huang J, Jaske E, Paulsen D, Jones C, La Crosse encephalitis in eastern Tennessee: evidence of invasive mosquito (Aedes albopictus and Ochlerotatus japonicus) involvement in the transmission of an indigenous disease. Am J Trop Med Hyg. 2011;85(suppl):374 [cited 2014 Feb 2]. http://www.ajtmh.org/content/85/6_Suppl/351.full.pdf+html?sid=394382c5-4458-4fe8-81b9-a0ad7f0b8628
- Molaei G, Farajollahi A, Scott JJ, Gaugler R, Andreadis TG. Human bloodfeeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. J Am Mosq Control Assoc. 2009;25:210–4. DOIPubMedGoogle Scholar
- Kaufman MG, Fonseca DM. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu Rev Entomol. 2014;59:31–49 . DOIPubMedGoogle Scholar
- Haddow AD, Bixler D, Odoi A. The spatial epidemiology and clinical features of reported cases of La Crosse virus infection in West Virginia from 2003 to 2007. BMC Infect Dis. 2011;11:29. DOIPubMedGoogle Scholar
- Lambert AJ, Nasci RS, Cropp BC, Martin DA, Rose BC, Russell BJ, Nucleic acid amplification assays for detection of La Crosse virus RNA. J Clin Microbiol. 2005;43:1885–9. DOIPubMedGoogle Scholar
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis, version 2.73. 2010 [cited 2014 Dec 16]. http://mesquiteproject.org
- Wheeler TJ, Kececioglu JD. Multiple alignments by aligning alignments. Bioinformatics. 2007;23:i559–68. DOIPubMedGoogle Scholar
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–90. DOIPubMedGoogle Scholar
- Armstrong PM, Andreadis TG. A new genetic variant of La Crosse virus (Bunyaviridae) isolated from New England. Am J Trop Med Hyg. 2006;75:491–6 .PubMedGoogle Scholar
Figures
Table
Cite This Article1Current affiliation: United States Geological Survey, Reston, Virginia, USA.
Table of Contents – Volume 21, Number 4—April 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
M. Camille Harris, US Geological Survey, Office of Ecosystems, 12201 Sunrise Valley Dr, Reston, VA 20192, USA
Top