Volume 21, Number 7—July 2015
CME ACTIVITY - Research
Parechovirus Genotype 3 Outbreak among Infants, New South Wales, Australia, 2013–2014
Cite This Article
Citation for Media
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; (4) view/print certificate.
Release date: June 15, 2015; Expiration date: June 15, 2016
Learning Objectives
Upon completion of this activity, participants will be able to:
1. Describe the clinical and epidemiologic features of an HPeV3 outbreak among Australian infants
2. Compare the presentation of the HPeV3 outbreak in Australia with that in the northern hemisphere
3. Determine the efficacy of active surveillance in detecting and monitoring the HPeV3 outbreak among Australian infants.
CME Editor
P. Lynne Stockton, VMD, MS, ELS(D), Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: P. Lynne Stockton, VMD, MS, ELS(D), has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Germaine Cumming, MPHN; Ameneh Khatami, BHB, MB ChB, DipPaeds, MD; Brendan J. McMullan, BMed(Hons), DTMH, DCH, FRACP, FRCPA; Jennie Musto, Dip.App.Sci (Nursing), Grad.Dip.Clinical Nursing, MPH; Kit Leung, MIPH; Oanh Nguyen, MD, MCH; Mark J. Ferson, MBBS, MPH, MD, FRACP, FAFPHM, FRSPH; Georgina Papadakis, BSc; and Vicky Sheppeard, MBBS, MPH(Hons), FAFPHM, have disclosed no relevant financial relationships.
Abstract
From October 2013 through February 2014, human parechovirus genotype 3 infection was identified in 183 infants in New South Wales, Australia. Of those infants, 57% were male and 95% required hospitalization. Common signs and symptoms were fever >38°C (86%), irritability (80%), tachycardia (68%), and rash (62%). Compared with affected infants in the Northern Hemisphere, infants in New South Wales were slightly older, both sexes were affected more equally, and rash occurred with considerably higher frequency. The New South Wales syndromic surveillance system, which uses near real-time emergency department and ambulance data, was useful for monitoring the outbreak. An alert distributed to clinicians reduced unnecessary hospitalization for patients with suspected sepsis.
The clinical manifestations of infection with human parechoviruses (HPeVs), members of the family Picornaviridiae, are often indistinguishable from those caused by human enterovirus infections. Over the past decade, outbreaks of human parechovirus genotype 3 (HPeV3) have been reported from the Northern Hemisphere and are particularly well documented in Japan (where the virus was discovered), Canada, the United Kingdom, Denmark, and the Netherlands (1–4). Of the 16 HPeV genotypes, HPeV3 is the most aggressive and causes a sepsis-like syndrome in neonates (5). HPeV infection seems to follow a seasonal pattern; incidence is higher in summer and autumn (2,3). It can be spread by the fecal–oral and respiratory routes (4).
On November 22, 2013, Health Protection New South Wales (NSW), Australia, was notified of a possible cluster of HPeV cases at The Children’s Hospital at Westmead in Sydney. At that time, 7 neonates had experienced rapid onset of acute sepsis-like illness with fever >38°C and a combination of irritability/pain, diarrhea, confluent erythematous rash, tachycardia, tachypnea, encephalitis, myoclonic jerks, and hepatitis. Inquiries revealed that neonates described as “red, hot, angry” had also been admitted to other tertiary children’s hospitals in NSW (6). An expert advisory group comprising staff from the NSW Ministry of Health, Health Protection NSW, public health units, and the Sydney Children’s Hospital Network was convened to coordinate the investigation.
On November 25, 2013, PCR detection of HPeV RNA confirmed HPeV infection in 2 of the children. The NSW public health network and clinicians agreed that a surveillance program should be initiated to gather information on the epidemiologic and clinical characteristics and outcomes of children with HPeV infection.
In addition to the public health response, Health Protection NSW issued a media release to alert members of the public to the outbreak. On November 29, 2013, HPeV3 information including a case definition, instructions for accessing diagnostic testing, and recommended clinical management was distributed to all emergency departments, pediatricians, and early childhood health services in NSW. During the outbreak, the expert advisory group met regularly via teleconference to discuss and address any emerging issues. HPeV3 active surveillance activities were concluded on January 31, 2014, while other forms of surveillance continued into February 2014. We describe the epidemiology of the outbreak as observed through several surveillance mechanisms.
HPeV infection is not a notifiable disease under the Public Health Act 2010 (NSW). This HPeV3 outbreak was detected and reported by clinicians alert to unusual clusters and patterns of disease. Other forms of surveillance were developed as a result of this alert. Surveillance consisted of 3 components: 1) active surveillance (case finding at the sentinel sites); 2) passive surveillance (laboratory reporting of all positive HPeV specimens from sentinel sites and elsewhere in NSW to Health Protection NSW); and 3) syndromic surveillance (reporting of infants seen in emergency departments by the NSW syndromic surveillance system that uses near real-time emergency department and ambulance data [7]) (Figure 1). The sentinel sites were 3 tertiary children’s hospitals in NSW: The Children’s Hospital at Westmead, The Sydney Children’s Hospital Randwick, and John Hunter Children’s Hospital Newcastle. Passive and syndromic surveillance continued into February 2014. In addition to surveillance, public health communication in the form of an HPeV information sheet for clinicians was distributed on November 29, 2013, alerting emergency department staff, pediatricians, and early childhood health service staff of current HPeV activity in NSW, providing a description of HPeV infection, and recommending management options (i.e., early laboratory testing and provision of supportive care after receipt of confirmation of HPeV infection).
Active Surveillance
Active surveillance activities commenced at the 3 hospitals (sentinel sites) on November 25, 2013, and continued through January 19, 2014; however, some retrospective case finding was included for cases with onset dating back to October 1, 2013, when the outbreak was thought to have started. A patient with a suspected case of HPeV was defined as a neonate or young infant <3 months of age with sepsis-like illness and fever >38.0°C and >2 of the following: irritability/pain, rash, diarrhea, tachycardia, tachypnea, encephalitis, myoclonic jerks, or hepatitis. A patient with a confirmed case of HPeV was a suspected case-patient for whom PCR was positive for HPeV. Clinicians at the sentinel sites collected case information by using an HPeV case investigation form and PCR testing of patient stool, cerebrospinal fluid (CSF), nasopharyngeal aspirate, throat swab, rectal swab, or whole blood samples for HPeV; stool and CSF samples were preferred. (3,8,9). These data were entered into the NSW Notifiable Conditions Information Management System. Weekly reports informed the NSW public health network of outbreak progression.
Passive Surveillance
All positive HPeV test results from NSW residents referred to the Victorian Infectious Diseases Reference Laboratory (VIDRL) in Melbourne, Victoria (the only laboratory in the region testing for HPeV), from October 1, 2013, through February 2, 2014, were reported to NSW Health. Specimen date; estimated illness onset date; sample type; and patient date of birth, sex, and postcode were recorded in the NSW Notifiable Conditions Information Management System.
Syndromic Surveillance
The NSW emergency department syndromic surveillance system monitored the number of infants <1 year of age for whom a provisional diagnosis of fever/unspecified infection was made in the emergency department and the number of patients who required hospital admission, including admission to critical care wards. A diagnosis of fever/unspecified infection can include fever symptoms, unspecified viral infection, unspecified viremia, unspecified bacteremia, unspecified bacterial infection, or unspecified infection. Weekly reports compared recent data with historical data from the previous 5 years.
Laboratory Methods
From all clinical samples, nucleic acid was extracted by using QIAGEN DX reagents (QIAGEN, Hilden, Germany) on a QIAxtractor NA extraction robot (QIAGEN). cDNA was synthesized by using a method previously described (10) and was tested in an HPeV real-time PCR selective for the 5′ untranslated region, which was developed at VIDRL (10). (The primer and probe sequence details for this assay can be supplied upon request to G.C.)
Molecular analysis to obtain the HPeV genotype was performed on selected samples that had been positive by real-time PCR. Specimens from 41 patients were selected for genotyping on the basis of ensuring representation of infants’ geographic locations, ages, sex, illness onset dates, specimen types, and sex. Identification of specific HPeV genotypes was obtained through amplification of the viral protein 1 gene by use of a gel-based seminested PCR (11). The generated PCR products were sequenced and compared with reference sequences by using the primers and methods described elsewhere (12).
Statistical Analyses
Descriptive analysis of epidemiologic variables and patient demographic characteristics were performed. Characteristics of infants <3 months of age, such as length of stay (LOS), were compared by using t-tests to determine the effects of public health messaging. Analyses was performed by using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA).
Laboratory Findings
From November 1, 2013, through February 28, 2014, a total of 420 specimens were submitted for HPeV PCR testing; for some patients, >1 specimen was submitted. PCR results were HPeV positive for 289 (69%) specimens from 198 patients (Table 1). In addition to confirming HPeV RNA in samples from 198 patients in NSW, HPeV type 3 was identified from all 41 (21%) positive samples for which molecular analysis was subsequently performed. The phylogenetic tree demonstrating all HPeV3 isolates genotyped at VIDRL during the outbreak is reported elsewhere (6).
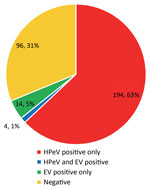
Because of the algorithms used in the testing, enterovirus results were also available for all samples submitted (Table 1). A total of 194 patients had HPeV infection only, 4 had dual infections (HPeV and enterovirus), and 14 had enterovirus infection only (Figure 2). Results for the rest of the patients were negative. Focusing on CSF and fecal samples, 123 (73%) of 168 CSF samples were HPeV positive by PCR (mean cycle threshold [Ct] detection value 31.6), and 114 (73%) of 156 fecal samples were positive (mean Ct 27.2) (Table 2). PCR was run for 45 cycles; therefore, Ct values >45 were considered negative.
Active and Passive Surveillance Findings
Active surveillance identified 94 infants whose illness met the definition of a confirmed case (patient <3 months of age and HPeV-positive laboratory results, originating from sentinel sites). Passive surveillance spanning specimen collection dates from October 1, 2013, through February 2, 2014, identified another 89 laboratory-confirmed cases in NSW in patients 0–17 months of age. The outbreak peaked during the first 2 weeks of December 2013 (Figure 3).
More cases (105 [57%]) were in male than female patients; median patient age was 1.51 months (or median 46 days, range 0–537 days) (Figure 4; Table 3). Intrafamily HPeV3 transmission was identified in twins, 2 parent–child pairs, and a set of cousins. A descriptive case series of the infants infected with HPeV3 during this outbreak, containing further clinical details on select cases, is reported elsewhere (6).
Analysis of case investigation forms from the sentinel sites reporting HPeV signs and symptoms showed that the most commonly reported signs for infants <3 months of age were fever (86%), irritability/pain (80%), tachycardia (68%), and rash (62%) (Table 3). Similar signs were displayed by those >3 months of age; however, 20% fewer in this age group had fever and tachycardia (Table 3). As described previously, all infants at the sentinel sites were well at the time of hospital discharge, and further longitudinal follow-up studies are examining the long-term outcomes of these infections having occurred in early life (6).
Of the 183 confirmed cases, 108 (59%) were captured by the 3 sentinel surveillance sites, and another 75 (41%) were diagnosed at other hospitals. Most (57%) patients resided in the Sydney metropolitan area, and the remaining 43% were from regional or rural areas of NSW. This finding compares with 64% and 36% of the NSW population residing in metropolitan Sydney and regional/rural areas, respectively (13).
Analysis of case investigation forms for the 108 patients at the sentinel sites also showed that 103 (95%) patients were admitted to hospital and had an average LOS of 4.4 (1–13) days (Table 3). Mean LOS was greater for infants <3 months of age (4.5, range 1–13 days) than for those >3 months of age (3.7, range 1–11 days). The rate of admission to an intensive care unit was lower for older infants (14%) than for those <3 months of age (30%) (Table 3).
The trend statistic on the distribution of LOS in infants <3 months of age showed that LOS was significantly reduced among infants <3 months of age who became ill after the HPeV alert was sent on November 29, 2013, to emergency departments and pediatricians. Mean LOS at the sentinel sites was 5.7 days before and 4.0 days after sending of the alert (Satterthwaite t-test, p<0.05) (Figure 5).
Syndromic Surveillance Findings
The number of infants <1 year of age with a provisional emergency department diagnosis of fever/unspecified requiring hospital admission began to rise sharply in mid-November 2013 and peaked during the first week of December (Figure 6, panel A). At the peak, the number of admissions was 83, compared with an average of 52 for the same week in previous years. Admissions remained elevated until mid-January 2014, when they returned to background levels. Admissions to critical care wards spiked during the second week of December, when 9 patients were admitted, well above the average of 1 admission per week for the same week in previous years (Figure 6, panel B). During the surveillance period, most admissions were to the 2 children’s hospitals in metropolitan Sydney.
This outbreak is probably one of the first large parechovirus outbreaks to be reported in Australia. We observed a large number of cases over a relatively short period (≈4 months), peaking around late spring/early summer, which is earlier than documented seasonality for parechovirus in the Northern Hemisphere (3). Although sequencing in this series was incomplete (21% of patients), this parechovirus outbreak was determined to have been caused by HPeV3 for the following reasons: all sequenced HPeV-positive samples were genotype 3; the epidemiology and spectrum of illness seen by clinicians at the sentinel sites was relatively homogenous and in keeping with other reports of HPeV3 infections in infants (3,14); and PCR was enterovirus positive for only 18 patients but HPeV positive for 198 patients.
Infected infants in this outbreak were older than those reported elsewhere. The median age of 46 days was higher than that reported in Denmark (39 days) and the Netherlands (40 days) (3,14). The occurrence of a substantial proportion (17%) of HPeV3 infections in infants >3 months of age was consistent with results from a US study reporting 18% of cases in infants >60 days of age but contrary to other data indicating that HPeV3 infection occurs almost exclusively in infants <3 months of age (2,4).
The age cutoff in the case definition for active surveillance at the sentinel sites may have introduced a bias toward HPeV infection being more frequently suspected in infants <3 months of age; thus, these infants might have undergone more HPeV testing than older infants, thereby underestimating the true mean age of infants affected in this outbreak. Had the age cutoff in the case definition been set at 12 months (all infants), bias toward younger infants would have been avoided, and the median age might have been older than that reported here.
After the November 29, 2013, release of the HPeV alert to emergency department staff and pediatricians, mean LOS among infants <3 months of age decreased by 30%, from ≈5.7 days at the start of the outbreak to 4.0 days (p = 0.0250). This statistically significant finding reflects a degree of effectiveness of public health messaging in raising clinician awareness of HPeV. During the latter part of the outbreak, after Health Protection NSW issued the alert, informed clinicians may have felt more comfortable discontinuing treatment and discharging infants sooner if their illness met the criteria of the HPeV case definition. The alert seemed to result in improved clinical management of cases, avoidance of unnecessary prolonged exposure to empirically prescribed antimicrobial drugs, provision of appropriate supportive treatment, and earlier discharge from hospital. The mean LOS during the NSW outbreak (4.4 days) was lower than that reported in the Netherlands (7 days), according to an analysis of retrospective diagnoses when no public health intervention would have taken place (8). Another factor that could contribute to reduced LOS in the latter part of an outbreak is the increasing age of young infants becoming ill with HPeV infection in the second half of the outbreak, although this factor was not statistically significant in this dataset (15).
The male:female split in this outbreak was less pronounced than that reported for other studies, although more cases consistently occurred in boys. Others have reported a higher preponderance of infection in boys, ranging from 70% to 90%, compared with our finding of 57% (2,5,8,14).
Clinical signs reported in the literature for HPeV3-infected infants were consistent with our findings of fever, irritability, and encephalitis (2,6,8). However, rash occurred with much higher frequency (62% vs. 17%) in the NSW outbreak than in other outbreaks (8).
Syndromic surveillance that used emergency department data proved to be a useful and timely way to monitor emergency department hospital admissions temporally associated with the HPeV3 outbreak. Increased presentation of infants <1 year of age with a provisional emergency department diagnosis of fever/unspecified infection requiring hospital admission, in particular admission to critical care, were associated with increased detection of HPeV3 at the clinical level. Emergency department syndromic surveillance reports also helped confirm an overall decline in admissions from emergency departments in early 2014, supporting the eventual withdrawal of active surveillance. Emergency department syndromic surveillance in NSW does not routinely monitor age-specific or admission-specific (hospital ward or critical care unit) aberrations; that is, all children <5 years of age are monitored as a group. In the future, data generated through the emergency department syndromic surveillance system may continue to be useful for monitoring the evolution of an HPeV or similar outbreak. The cost of maintaining emergency department syndromic surveillance like that used during this outbreak (fever/unspecified infection among children <1 year of age and admission to hospital) would need to be considered. Costs of doing so include personnel time for checking reports and investigating signals, infrastructure costs, and opportunity costs; choosing to monitor HPeV3-related signals indirectly means that signals for diseases that are not prioritized are not monitored. In addition, the sensitivity of this grouping will need to be tested in future outbreaks before it can be considered a reliable proxy indicator of a seasonal outbreak
We initiated sentinel surveillance on the assumption that nearly all neonates with severe HPeV3 disease would be referred to 1 of the 3 tertiary children’s hospitals in NSW. Through passive surveillance we identified an additional 41% of HPeV patients who had been seen at other health facilities. Enhanced data were not collected for these presumably milder cases, which has limited our capacity to conduct more extensive significance testing across the observed differences in clinical features. Recording more information on potential exposures (e.g., infants’ daycare attendance, existence of older siblings, and occurrence of family illness in weeks preceding infants’ admission) would have further aided our understanding of HPeV3 transmission in the community.
The objectives of HPeV surveillance were achieved: document the outbreak, describe the clinical features of cases, help inform clinicians and the public, monitor the evolution of the outbreak, and add to the knowledge base. The HPeV3 infection outbreak in NSW, Australia, differed slightly from that documented in the Northern Hemisphere; the NSW outbreak apparently affected slightly older infants (as well as neonates and young infants), cases were more evenly split between boys and girls, and rash occurred at a considerably higher frequency. The value of awareness-raising communication strategies was demonstrated by the statistically significant 30% reduction in LOS during the outbreak immediately after release of the alert to emergency department staff and pediatricians. This alert helped to minimize unneeded use of antimicrobial drugs and reduce unnecessary hospitalization. Although active surveillance is resource intensive, it has helped to define HPeV3 infection in NSW and link it with a syndromic surveillance indicator in the emergency department syndromic surveillance system. Syndromic surveillance is a potentially useful proxy indicator that should be considered for future detection and surveillance of seasonal outbreaks of viral infections.
Ms. Cumming is a public health specialist working with the NSW health system. Her research areas of interest include population health surveillance and evaluations and public health response preparedness.
.
Acknowledgments
We express our gratitude to clinicians at the sentinel sites and laboratory staff at South Eastern Areas Laboratory Services, Children’s Hospital Westmead Pathology, and Hunter Area Pathology Service. Particular thanks go to Stephanie Francis and Emma Goeman, Murray Webber, and Karin Timmers for their assistance with data collection and reporting to Public Health. We also thank the staff at the public health units for managing case information and laboratory results in the NSW Notifiable Conditions Information Management System, particularly Margaret Osbourn, Julie K. Kohlhagen, and Gareth Hockey. We thank the Ministry of Health staff, particularly Tracie Reinten, for statistical analysis contributions, and David Muscatello and Tina Navin-Cristina for continued weekly syndromic surveillance of HPeV infection in infants in NSW. We are very grateful for the knowledge, expertise, and advice received from our Expert Panel members, particularly Sheena Adamson, Stephen Corbett, David Durrheim, Alison Kesson, Tony Merritt, and Murray Webber. We appreciate the vital support received from VIDRL, particularly Julian Druce, for an excellent speedy turnaround of HPeV testing and genotyping, which was not available in NSW at the time.
This work was completed while G.C. was employed as a trainee in the NSW Public Health Officer Training Program funded by the NSW Ministry of Health.
References
- Selvarangan R, Nzabi M, Selvaraju SB, Ketter P, Carpenter C, Harrison CJ. Human parechovirus 3 causing sepsis-like illness in children from Midwestern United States. Pediatr Infect Dis J. 2011;30:238–42. DOIPubMedGoogle Scholar
- Sharp J, Harrison CJ, Puckett K, Selvaraju SB, Penaranda S, Nix WA, Characteristics of young infants in whom human parechovirus, enterovirus or neither were detected in cerebrospinal fluid during sepsis evaluations. Pediatr Infect Dis J. 2013;32:213–6 .PubMedGoogle Scholar
- Fischer TK, Midgley S, Dalgaard C, Nielsen AY. Human parechovirus infection, Denmark. Emerg Infect Dis. 2014;20:83–7. DOIPubMedGoogle Scholar
- Harvala H, Wolthers KC, Simmonds P. Parechovirus in children: understanding a new infection. Curr Opin Infect Dis. 2010;23:224–30. DOIPubMedGoogle Scholar
- Guo Y, Duan Z, Qian Y. Changes in human parechovirus profiles in hospitalised children with acute gastroenteritis after a three-year interval in Lanzhou, China. PLoS ONE. 2013;8:e68321. DOIPubMedGoogle Scholar
- Khatami A, McMullan B, Webber M, Stewart P, Francis S, Timmers K, Sepsis-like disease in infants due to human parechovirus type 3 during an outbreak in Australia. Clin Infect Dis. 2015;60:228–36. DOIPubMedGoogle Scholar
- Muscatello DJ, Churches T, Kaldor J, Zheng W, Chiu C, Correll P, An automated, broad-based, near real-time public health surveillance system using presentations to hospital emergency departments in New South Wales, Australia. BMC Public Health. 2005;5:141. DOIPubMedGoogle Scholar
- Wolthers KC, Benschop KS, Schinkel J, Molenkamp R, Bergevoet RM, Spijkerman IJ, Human parechovirus as an important viral cause of sepsis-like illness and meningitis in young children. Clin Infect Dis. 2008;47:358–63. DOIPubMedGoogle Scholar
- Shoji K, Komuro H, Miyata I, Miyairi I, Saitoh A. Dermatologic manifestations of human parechovirus type 3 infection in neonates and infants. Pediatr Infect Dis J. 2013;32:233–6 .PubMedGoogle Scholar
- Druce J, Tran T, Kelly H, Kaye M, Chibo D, Kostecki R, Laboratory diagnosis and surveillance of human respiratory viruses by PCR in Victoria, Australia, 2002–2003. J Med Virol. 2005;75:122–9. DOIPubMedGoogle Scholar
- Nix WA, Maher K, Pallansch MA, Oberste MS. Parechovirus typing in clinical specimens by nested or semi-nested PCR coupled with sequencing. J Clin Virol. 2010;48:202–7. DOIPubMedGoogle Scholar
- Papadakis G, Chibo D, Druce J, Catton M, Birch C. Detection and genotyping of enteroviruses in cerebrospinal fluid in patients in Victoria, Australia, 2007–2013. J Med Virol. 2014;86:1609–13 . DOIPubMedGoogle Scholar
- Australian Bureau of Statistics. Estimated resident population (ERP) by region, age, and sex, 2001 to 2013 [cited 2014 Feb 24]. http://www.abs.gov.au/.
- Benschop KS, Schinkel J, Minnaar RP, Pajkrt D, Spanjerberg L, Kraakman HC, Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin Infect Dis. 2006;42:204–10. DOIPubMedGoogle Scholar
- Lenski RE, May RM. The evolution of virulence in parasites and pathogens: reconciliation between two competing hypotheses. J Theor Biol. 1994;169:253–65. DOIPubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the “Register” link on the right hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/about-ama/awards/ama-physicians-recognition-award.page. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Parechovirus Genotype 3 Outbreak among Infants, New South Wales, Australia, 2013–2014
CME Questions
1. Your patient is an 18-month-old Australian boy presenting with fever and presumed sepsis. According to the surveillance report by Cumming and colleagues, which of the following statements about the clinical and epidemiologic features of a human parechovirus genotype 3 (HPeV3) outbreak among Australian infants is correct?
A. Between October 2013 and February 2014, a total of 183 cases of HPeV3 were identified in NSW infants
B. Three-quarters of affected infants were girls
C. Half of affected infants required hospitalization
D. Common symptoms were diarrhea, sleepiness, and cough
2. According to the surveillance report by Cumming and colleagues, which of the following statements about the presentation of the HPeV3 outbreak in Australia compared with that in the northern hemisphere is correct?
A. The Australian outbreak affected slightly younger infants
B. The Australian outbreak had a more even gender split
C. Frequency of skin rash was lower in the Australian outbreak
D. Peak number of cases was later than documented in the northern hemisphere
3. According to the surveillance report by Cumming and colleagues, which of the following statements about the efficacy of active surveillance in detecting and monitoring the HPeV3 outbreak among Australian infants would most likely be accurate?
A. Syndromic surveillance was not useful for outbreak monitoring
B. Public health response had no apparent effect on infant length of stay
C. Awareness-raising communication strategies were ineffective
D. Active surveillance is resource intensive but helped to define the infection and link it with a syndromic surveillance indicator
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
1Current affiliation: Independent consultant, Freshwater, New South Wales, Australia
Related Links
Table of Contents – Volume 21, Number 7—July 2015
| EID Search Options |
|---|
|
|
|
|
|
|





Please use the form below to submit correspondence to the authors or contact them at the following address:
Germaine Cumming, 20 Wyuna Ave, Freshwater, NSW 2096, Australia
Top