Volume 21, Number 7—July 2015
CME ACTIVITY - Synopsis
Disseminated Infections with Talaromyces marneffei in Non-AIDS Patients Given Monoclonal Antibodies against CD20 and Kinase Inhibitors
Cite This Article
Citation for Media
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; (4) view/print certificate.
Release date: June 15, 2015; Expiration date: June 15, 2016
Learning Objectives
Upon completion of this activity, participants will be able to:
1. Distinguish the clinical and epidemiologic characteristics of T. marneffei infection, based on a case series report
2. Discuss the recent emergence of disseminated T. marneffei infection in non-AIDS patients with hematologic malignant neoplasms treated with targeted therapies
3. Identify possible mechanisms of action underlying disseminated T. marneffei infection in non-AIDS patients with hematologic malignant neoplasms treated with targeted therapies
CME Editor
Thomas J. Gryczan, MS, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Thomas J. Gryczan, MS, has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Harinder Gill, MBBS; Frank Y.F. Lam, MBBS, MRCP(UK), FHKAM; Nigel J. Trendell-Smith, MBBS, FRCPath; Siddharth Sridhar, MBBS, MRCP(UK); Herman Tse, MBBS; Susanna K.P. Lau, MD; and Kwok-Yung Yuen, MD, have disclosed no relevant financial relationships. Jasper F.W. Chan, MBBS, FRCPath, FRCP(Edin), has disclosed the following relevant financial relationships: received travel grants from Pfizer Co. HK Ltd. and Astellas Pharma HK Co. Ltd. Thomas S.Y. Chan, MBBS(Hons), MRCP(UK), FHKAM(Med), has disclosed the following relevant financial relationships: served as a speaker or a member of a speakers bureau for Pfizer. Ivan F.N. Hung, MD, has disclosed the following relevant financial relationships: served as an advisor or consultant for Pfizer (Asia Pacific Capital Advisory Board), MSD; received conference sponsorships from AstraZeneca, Ferring. Patrick C.Y. Woo, MD, has disclosed the following relevant financial relationships: involved in Tigecycline Evaluation Surveillance Trial with Pfizer.
Abstract
Infections with the fungus Talaromyces (formerly Penicillium) marneffei are rare in patients who do not have AIDS. We report disseminated T. marneffei infection in 4 hematology patients without AIDS who received targeted therapy with monoclonal antibodies against CD20 or kinase inhibitors during the past 2 years. Clinicians should be aware of this emerging complication, especially in patients from disease-endemic regions.
Talaromyces (formerly Penicillium) marneffei is a pathogenic, thermal dimorphic fungus that causes systemic mycosis in Southeast Asia. T. marneffei infection is characterized by fungal invasion of multiple organ systems, especially blood, bone marrow, skin, lungs, and reticuloendothelial tissues, and is highly fatal, especially when diagnosis and treatment are delayed (1,2). This disease is found predominantly in AIDS patients and occasionally those with cell-mediated immunodeficiencies involving the interleukin-12/interferon-γ (IFN-γ) signaling pathway, such as congenital STAT1 mutations or acquired autoantibodies against IFN-γ (1,3–6). The infection has rarely been reported among hematology patients, including those from disease-endemic regions (7,8).
At Queen Mary Hospital in Hong Kong, a 1,600-bed university teaching hospital that has a hematopoietic stem cell transplantation service, where a wide range of invasive fungal infections have been observed (9,10), only 3 cases of T. marneffei infection were encountered in >2,000 hematology patients in the past 20 years, despite the long-standing availability of mycologic culture and serologic testing (7,8,11,12). In contrast, the infection was commonly reported among AIDS patients (13).
In the past 2 years, we have been alerted by 4 unprecedented cases of disseminated T. marneffei infection among non-AIDS hematology patients given targeted therapies, including monoclonal antibodies (mAbs) against CD20 and kinase inhibitors, which are being increasingly used in recent years. We report details for these 4 hematology case-patients. The study was approved by the institutional review board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster in Hong Kong.
Patient 1 was a 56-year-old Filipino man with Waldenström macroglobulinemia, idiopathic thrombocytopenic purpura, and primary biliary cirrhosis. He had fever, night sweating, productive cough, and left facial pain for 1 week and bloody diarrhea for 2 days. He had previously received fludarabine, dexamethasone, and rituximab (mAb against CD20, 18 months earlier) for treatment of Waldenström macroglobulinemia (Table 1). The idiopathic thrombocytopenic purpura was controlled with intravenous immunoglobulin and maintenance prednisolone and mycophenolate sodium. A chest radiograph showed a small cavitary lesion in the right lower lobe. His symptoms and signs did not resolve after he received empirical intravenous imipenem/cilastatin and metronidazole (Table 2).
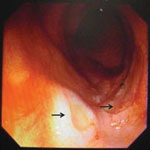
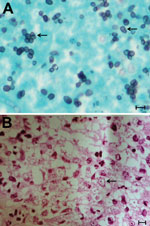
A colonoscopy showed multiple shallow ulcers at the terminal ileum (Figure 1). Histologic analysis of an ulcer biopsy specimen showed slough of an acutely inflamed ulcer but no microorganisms. However, histologic analysis of a specimen from a nasopharyngeal biopsy performed for persistent left facial pain showed abundant yeast cells engulfed by foamy macrophages (Figure 2). Culture of terminal ileal ulcer biopsy specimens, stool samples, and nasopharyngeal biopsy specimens yielded T. marneffei. A contrast-enhanced cranial computed tomography (CT) scan showed 2 lesions (3–4-mm) with rim enhancement and perifocal edema at the right occipital and left parieto-occipital lobes. A thoracic CT scan showed 2 cavitary lesions (4–8 mm) in the right upper and lower lobes.
Immunologic testing showed that the patient was negative for HIV and autoantibodies against IFN-γ. His CD3+ and CD8+ counts were within references ranges, but he had mild CD4+ lymphopenia (Table 2). His fever and symptoms resolved with after 2 weeks of treatment with intravenous liposomal amphotericin B, followed by oral voriconazole. Reassessment colonoscopy (at 2 months) and CT scan (at 6 months) showed complete resolution of all lesions.
Patient 2 was a 44-year-old Chinese man who had fever for 2 days. He had previously received chemotherapy and mAbs against CD20 (rituximab, 14 months earlier; obinutuzumab, concomitant) for refractory chronic lymphocytic leukemia (CLL) involving bone marrow (Table 1). He was empirically given intravenous piperacillin/tazobactam and anidulafungin (Table 2). Histologic analysis of a trephine biopsy specimen showed persistent CLL with plasmacytic differentiation, and Grocott staining showed yeasts with central septa in small clusters. Culture of peripheral blood and bone marrow aspirate yielded T. marneffei. A change in antifungal treatment to intravenous amphotericin B led to defervescence and clearance of fungemia. He was given oral itraconazole as maintenance therapy. He remained well until 2 months later when he was hospitalized for deteriorating CLL complicated by neutropenic fever with multiorgan failure caused by other opportunistic infections (Table 1). He died 5 months after the episode of disseminated T. marneffei infection.
Patient 3 was a 63-year-old Chinese man with myelofibrosis and well-controlled diabetes mellitus. He had intermittent fever, right cervical lymphadenopathy, and productive cough for 4 months. He was given ruxolitinib (kinase inhibitor) 6 months before symptom onset because of transfusion-dependent myelofibrosis despite splenectomy 4 years earlier (Table 1). A chest radiograph and thoracic CT scan showed multiple cavitary lesions and consolidation. Bronchoalveolar lavage was negative for bacteria, fungi, and mycobacteria. A serum cryptococcal antigen test result was negative. He was empirically given intravenous imipenem/cilastatin and oral doxycycline, but his symptoms persisted. A right cervical lymph node culture yielded T. marneffei. His symptoms and radiologic abnormalities resolved after treatment with intravenous amphotericin B for 2 weeks, followed by oral voriconazole for 6 months.
Patient 4 was a 67-year-old Chinese man with acute myeloid leukemia and hypertension. He had fever and malaise for 2 days without localizing signs. He had been given sorafenib (kinase inhibitor) 8 months earlier for chemotherapy-refractory acute myeloid leukemia (Table 1). His fever did not respond to intravenous meropenem. Subsequently, 2 sets of blood cultures yielded T. marneffei. He was given intravenous amphotericin B for 2 weeks, followed by oral voriconazole. He remained well at follow-up 6 months after symptom onset.
T. marneffei infection is an emerging complication in hematology patients receiving targeted therapies. Historically, T. marneffei infection has rarely been seen in non-AIDS patients, even in disease-endemic regions. During 1994–2014, only 3 other cases were observed in our hematology patients (7,8,11). None of 47 patients with T. marneffei infection in another large local case series during 1994–2004 had hematologic disease (13). In the past 20 years, there has been no change in methods for laboratory diagnosis of T. marneffei infection or a marked increase in the number of hematology patients in our hospital. Therefore, these 4 cases indicate an increase in the incidence of T. marneffei infection in these patients. Although other immunosuppressants given to case-patients 1, 2, and 4 might have contributed to overall immunosuppression, none of these immunosuppressants, which have been used for years, have been associated with T. marneffei infection. Because use of targeted therapies is increasing in diverse patient groups, clinicians should be aware of this emerging complication, especially in patients from disease-endemic regions who have received these therapies with other immunosuppressants.
The exact mechanisms through which these targeted therapies lead to T. marneffei infection remain incompletely understood. Rituximab and obinutuzumab (used by case-patients cases 1 and 2) are mAbs against CD20 that predominantly target B cells. Unlike T cells, the role of B cell–mediated humoral response in T. marneffei infection is poorly defined. Although case-patient 1 had mild CD4+ lymphopenia probably related to concomitant use of prednisolone and mycophenolate sodium, T. marneffei infection is rarely seen in patients with CD4+ counts >300/µL (1). We postulate that B cell dysfunction might have impaired production of neutralizing antibodies against key virulence factors of T. marneffei or might involve impairment of cytokine-producing B cells, which are essential for T helper cell function (14).
More severe infections with fungemeia and bone marrow involvement developed in case-patients 2 and 4, who had undetectable levels of serum antibodies against T. marneffei. Correspondingly, case-patients 1 and 3, who had antibody titers >1:320, did not have positive blood culture results (Table 2). Symptoms developed in case-patient 1 more than a 1 year after he completed therapy with mAbs against CD20. This finding might be related to long-lasting B cell–depleting effects of mAbs against CD20 (15).
Regarding kinase inhibitors (used by cases-patients 3 and 4), ruxolitinib is a selective Janus kinase (JAK)-1/2 inhibitor that prevents signal transduction for type I/II cytokines, including IFN-γ, by interfering with the JAK-STAT signaling pathway. Use of ruxolitinib has been associated with opportunistic infections caused by intracellular pathogens, such as Mycobacterium tuberculsosis and Cryptococcus neoformans (16,17). Similarly, patients with impaired JAK-STAT signaling, but not those with diabetes mellitus or splenectomy (case-patient 3), are predisposed to T. marneffei infection (6). Sorafenib is a multikinase inhibitor with various immunomodulatory effects, including impaired T-cell response and proliferation and reduced IFN-γ production (18). These immune defects have been associated with reactivation of latent tuberculosis and might also predispose patients to opportunistic infections caused by intracellular organisms such as T. marneffei (18).
The recognition of disseminated T. marneffei infection as an emerging complication in non-AIDS patients treated with targeted therapy has major public health implications. In regions to which T. marneffei infection is endemic, serologic surveillance for patients receiving targeted therapy might be useful in the early diagnosis of T. marneffei infection, as in the case of AIDS patients (19). In non-endemic regions, such as the United States, clinicians should be vigilant of this infrequent infection in at-risk hematology patients who have resided in or are returning from disease-endemic areas.
Detailed information on case-patient 2 was also published in the January 2015 issue of the Annals of Hematology (20).
Dr. Jasper F.W. Chan is a clinical assistant professor in the Department of Microbiology, The University of Hong Kong, Hong Kong, China. His research interests include emerging infectious diseases and opportunistic infections in immunocompromised hosts.
Acknowledgment
This study was partly supported by donations from the Hui Hoy and Chow Sin Lan Charity Fund Limited; the Health and Medical Research Fund (ref. no. 13121342) and HKM-15-M07 (commissioned study) of the Food and Health Bureau of Hong Kong Special Administrative Region Government; the Strategic Research Theme Fund, The University of Hong Kong; and a Croucher Senior Medical Research Fellowship.
References
- Vanittanakom N, Cooper CR Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110. DOIPubMedGoogle Scholar
- Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, Phylogeny and nomenclature of the genus Talaromyces and taxa accomodated in Penicillium subgenus Biverticillium. Stud Mycol. 2011;70:159–83. DOIPubMedGoogle Scholar
- Tang BS, Chan JF, Chen M, Tsang OT, Mok MY, Lai RW, Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol. 2010;17:1132–8. DOIPubMedGoogle Scholar
- Chan JF, Trendell-Smith NJ, Chan JC, Hung IF, Tang BS, Cheng VC, Reactive and infective dermatoses associated with adult-onset immunodeficiency due to anti-interferon-gamma autoantibody: Sweet’s syndrome and beyond. Dermatology. 2013;226:157–66. DOIPubMedGoogle Scholar
- Lee PP, Chan KW, Lee TL, Ho MH, Chen XY, Li CH, Penicilliosis in children without HIV infection – are they immunodeficient? Clin Infect Dis. 2012;54:e8–19. DOIPubMedGoogle Scholar
- Lee PP, Mao H, Yang W, Chan KW, Ho MH, Lee TL, Penicillium marneffei infection and impaired IFN-γ immunity in humans with autosomal-dominant gain-of-phosphorylation STAT1 mutations. J Allergy Clin Immunol. 2014;133:8948–6.e5.
- Wong SS, Woo PC, Yuen KY. Candida tropicalis and Penicillium marneffei mixed fungaemia in a patient with Waldenstrom’s macroglobulinaemia. Eur J Clin Microbiol Infect Dis. 2001;20:132–5. DOIPubMedGoogle Scholar
- Woo PC, Lau SK, Lau CC, Chong KT, Hui WT, Wong SS, Penicillium marneffei fungaemia in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 2005;35:831–3. DOIPubMedGoogle Scholar
- Cheng VC, Chan JF, Ngan AH, To KK, Leung SY, Tsoi HW, Outbreak of intestinal infection due to Rhizopus microsporus. J Clin Microbiol. 2009;47:2834–43. DOIPubMedGoogle Scholar
- Yuen KY, Woo PC, Ip MS, Liang RH, Chiu EK, Siau H, Stage-specific manifestation of infection and impaired mold infections in bone marrow transplant recipients: risk factors and clinical significance of positive concentrated smears. Clin Infect Dis. 1997;25:37–42. DOIPubMedGoogle Scholar
- Wong SS, Wong KH, Hui WT, Lee SS, Lo JY, Cao L, Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. J Clin Microbiol. 2001;39:4535–40. DOIPubMedGoogle Scholar
- Yuen KY, Wong SS, Tsang DN, Chau PY. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344:444–5. DOIPubMedGoogle Scholar
- Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J. 2008;14:103–9 .PubMedGoogle Scholar
- Dang VD, Hilgenberg E, Ries S, Shen P, Fillatreau S. From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Curr Opin Immunol. 2014;28:77–83. DOIPubMedGoogle Scholar
- Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122:139–45. DOIPubMedGoogle Scholar
- Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor. Chest. 2013;143:1478–9. DOIPubMedGoogle Scholar
- Hopman RK, Lawrence SJ, Oh ST. Disseminated tuberculosis associated with ruxolitinib. Leukemia. 2014;28:1750–1. DOIPubMedGoogle Scholar
- Teo M, O’Connor TM, O’Reilly SP, Power DG. Sorafenib-induced tuberculosis reactivation. Onkologie. 2012;35:514–6. DOIPubMedGoogle Scholar
- Wang YF, Xu HF, Han ZG, Zeng L, Liang CY, Chen XJ, Serological surveillance for Penicillium marneffei infection in HIV-infected patients during 2004–2011 in Guangzhou, China. Clin Microbiol Infect. 2014;Dec 26:pii:S1198-743X(14)00167-0. Epub ahead of print.
- Tse E, Leung RY, Kwong YL. Invasive fungal infections after obinutuzumab monotherapy for refractory chronic lymphocytic leukemia. Ann Hematol. 2015;94:165–7.
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the “Register” link on the right hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/about-ama/awards/ama-physicians-recognition-award.page. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Disseminated Infections with Talaromyces marneffei in Non-AIDS Patients Given Monoclonal Antibodies against CD20 and Kinase Inhibitors
CME Questions
1. According to the case series report by Chan and colleagues, which of the following statements about the clinical and epidemiologic characteristics of Talaromyces marneffei infection is correct?
A. T. marneffei infection is the most important pathogenic thermal dimorphic fungus causing systemic mycosis in Southeast Asia
B. T. marneffei infection is typically limited to the gastrointestinal and/or urinary tract
C. T. marneffei infection is usually self-limited with a good prognosis for recovery
D. T. marneffei infection is common among non-AIDS hematology patients
2. Your patient is a 57-year-old Chinese man with acute myeloid leukemia and fever. According to the case series report by Chan and colleagues, which of the following statements about the recent emergence of disseminated T. marneffei infection in non-AIDS patients with hematologic malignant neoplasms treated with targeted therapies is correct?
A. The appearance of 4 cases in the past 2 years is the result of a change in the methodologies of a laboratory diagnosis of T. marneffei infection
B. The appearance of 4 cases in the past 2 years is the result of a dramatic increase in the number of hematology patients at the investigators' hospital
C. Recent emergence of disseminated T. marneffei infection is most likely because of overall immunosuppression
D. Recent emergence of disseminated T. marneffei infection is most likely because of targeted therapies, such as anti-CD20 monoclonal antibodies and kinase inhibitors
3. According to the case series report by Chan and colleagues, which of the following statements about possible mechanisms of action underlying disseminated T. marneffei infection in non-AIDS patients with hematologic malignant neoplasms treated with targeted therapies would most likely be accurate?
A. Rituximab and obinutuzumab used in 2 cases are anti-CD20 monoclonal antibodies that predominantly target T cells
B. T. marneffei infection is usually seen at CD4+ counts of more than 300/µL
C. Patients with B-cell dysfunction may have impaired production of neutralizing antibodies against key virulence factors of T. marneffei
D. The investigators do not suggest any role of cytokine-producing B-cells
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
Related Links
Table of Contents – Volume 21, Number 7—July 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Patrick C.Y. Woo, State Key Laboratory of Emerging Infectious Dieases, Department of Microbiology, Carol Yu Centre for Infection, The University of Hong Kong, University Pathology Bldg, Queen Mary Hospital Compound, Pokfulam Rd, Hong Kong, China
Top