Volume 21, Number 8—August 2015
Dispatch
Enterovirus A71 Meningoencephalitis Outbreak, Rostov-on-Don, Russia, 2013
Cite This Article
Citation for Media
Abstract
Seventy-eight cases of enterovirus infection, including 25 neuroinfections, occurred in Rostov-on-Don, Russia, during May–June 2013. The outbreak was caused by an enterovirus A type 71 (EV-A71) subgenotype C4 lineage that spread to neighboring countries from China ≈3 years earlier. Enterovirus associated neuroinfection may emerge in areas with a preceding background circulation of EV-A71 with apparently asymptomatic infection.
Enterovirus A type 71 (EV-A71; family Picornaviridae, genus Enterovirus, species Enterovirus A) is the most neuropathogenic nonpolio enterovirus in humans. Over the past 30 years, this virus has caused many outbreaks and epidemics of hand, foot and mouth disease (HFMD) with neurologic complications in the Asia-Pacific region and China (1). In Europe, EV-A71 commonly causes asymptomatic infection (2–4) and is only occasionally associated with severe neuroinfection (3,5). The last epidemics of EV-A71 infection in Europe occurred during 1975–1978 (6,7).
During 2013, an outbreak of EV-A71 infection occurred in Rostov-on-Don, a city in southern Russia. The first case was diagnosed on May 31 in a 2-year-old boy. A cerebrospinal fluid sample from the boy contained EV-A71 and pneumococcal bacteria, suggesting combined bacterial and viral meningoencephalitis. The boy died of meningoencephalitis on day 5 after disease onset. According to the official State Surveillance System report, 366 children (1–7 years of age) attended the same childcare facility as the boy who died, and over the 3 weeks after the index case, 77 more children were involved in the outbreak (Figure 1). Of the 78 children, 43 were boys and 35 were girls. Fourteen children were 1–2 years of age, 63 were 3–6 years of age, and 1 was 7 years of age. A total of 68 children were hospitalized, of whom 25 (15 boys, 10 girls) had meningitis or meningoencephalitis; 6 of these children were 1–2 years of age, and 19 were 3–6 years of age.
Enterovirus RNA was detected in fecal samples (n = 53), throat swab samples (n = 23), or both (n = 17) from 59 of the 78 patients. EV-A71 was identified by partial viral protein 1 genome region sequencing in the cerebrospinal fluid sample from the first patient and in fecal and throat swab samples from the other patients. The outbreak was officially defined as including only those children from the childcare facility attended by the index patient. Additional cases of HFMD and meningitis that occurred outside that kindergarten were not officially accounted for in outbreak statistics; thus, it is likely that the outbreak size was underestimated. Simovanian et al. (8) indicated that a total of 139 EV-A71 infections were reported in Rostov-on-Don during May–June 2013. According to that report, 30.2% of patients had meningitis and 7.2% had meningoencephalitis. Of note, exanthema was observed in only 79.9% of patients.
The ratio of neuroinfection (meningitis and meningoencephalitis) cases relative to mild infection (i.e., HFMD and fever) cases was unusually high in this outbreak. Among the 78 officially reported cases, 25 (32.1%) were meningitis or meningoencephalitis. After the first fatal case, all children in the childcare facility were proactively screened for enterovirus infection symptoms, so it is unlikely that many patients with mild infection were missed. Asymptomatic children were not screened for EV-A71 RNA. However, the ratio of meningitis cases was high even relative to the total number of children attending the childcare facility (6.8% [25/366 children]). This finding differs from those from a 1998 outbreak in Taiwan, in which 405 (0.3%) of 129,106 persons with HFMD had severe disease (i.e., fever >38°C or neurologic manifestations) (9). Thus, the proportion of severe disease in a localized setting can be substantially higher than that for an epidemic overall.
Thirteen isolates from the EV-A71–infected children were available for study. Viruses were propagated in cell culture, and the entire viral protein 1 genome region (891 nt) was amplified as described previously (10). The outbreak samples differed from each other by <3 nt substitutions (0.3%). Furthermore, they shared 97%–98% nt sequence identity with several subgenotype C4 viruses isolated in China during 2009–2011, indicating that the strains belonged to this subgenotype.
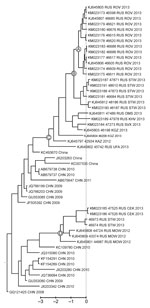
Phylogenetic analysis of the virus spread was performed by using a Bayesian phylogenetic approach implemented in BEAST version 1.7.5 (11). The substitution rate in this dataset was 6.8 × 10−3 substitutions per site per year, which is similar to rates reported for EV-A71 subgenogroup C4 in other studies (12,13). Sequences from Rostov-on-Don and the Rostov region were monophyletic (Figure 2, node A); the time to the most recent common ancestor was 200 days (95% high probability density interval 68–347 days); however, this group was imperfectly supported by a posterior probability value of 0.85. The high number of days to the most recent common ancestor of the outbreak strain is surprising because the outbreak presented as a point-source expansion with a traceable source and transmission chains. A possible explanation for this finding might be that that the substitution rate of EV-A71 might accelerate substantially during an outbreak, or additional substitutions might have been introduced upon isolation in cell culture.
In the phylogenetic tree, isolates from Rostov-on-Don grouped (with a posterior probability of 1) with an additional 6 viruses that were isolated in the neighboring Stavropol region during and up to 2 months after the outbreak (Figure 2, node B); however, no cases of neuroinfection were reported in that region. Isolates from Rostov-on-Don and Stavropol grouped reliably with another 7 viruses isolated in Siberia, the Pacific region of Russia, Kazakhstan, and Kyrgyzstan during 2012–2013 (Figure 2, node C). The time to the most recent common ancestor of these viruses was 921 days (95% high probability density range 665–1,195 days) before the outbreak. Therefore, the virus variant that caused the outbreak was likely introduced to Russia or Middle Asia ≈3 years before the outbreak and circulated extensively without causing notable illness. Of interest, another lineage of EV-A71 subgenogroup C4 representing an independent introduction from China was circulating at the same time in Central Russia and Stavropol region (Figure 2, node D).
Over the past 2 decades, EV-A71 became a prominent emerging virus in Asia. However, the EV-A71 epidemiologic situation remained calm in Russia, Europe, and North America, despite common circulation of the virus, as suggested by surveillance and seroepidemiologic studies (3,4). According to our previous study (10), EV-A71 was circulating in Rostov-on-Don before 2008, but EV-A71 seroprevalence among infants 1–2 years of age (4.5%) was substantially lower than that observed in other regions of Russia (9.9%–22.7%). It is possible that lower herd immunity could have facilitated the outbreak we describe here. Our findings show that an outbreak of EV-A71 neuroinfection may emerge in new areas despite preceding background circulation of EV-A71 with apparently asymptomatic infection.
Dr. Akhmadishina is a postdoctoral researcher. In 2013 she completed a PhD thesis on molecular epidemiology and seroepidemiology of EV-A71 in Russia.
Acknowledgment
This study received core funding from the participating institutions and from a Russian scientific foundation grant (no. 14-15-00619 to A.N.L. and L.V.A.).
References
- McMinn PC. Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Curr Opin Virol. 2012;2:199–205. DOIPubMedGoogle Scholar
- Schuffenecker I, Mirand A, Antona D, Henquell C, Chomel JJ, Archimbaud C, Epidemiology of human enterovirus 71 infections in France, 2000–2009. J Clin Virol. 2011;50:50–6. DOIPubMedGoogle Scholar
- Bible JM, Iturriza-Gomara M, Megson B, Brown D, Pantelidis P, Earl P, Molecular epidemiology of human enterovirus 71 in the United Kingdom from 1998 to 2006. J Clin Microbiol. 2008;46:3192–200. DOIPubMedGoogle Scholar
- Witsø E, Palacios G, Ronningen KS, Cinek O, Janowitz D, Rewers M, Asymptomatic circulation of HEV71 in Norway. Virus Res. 2007;123:19–29. DOIPubMedGoogle Scholar
- Vallet S, Legrand Quillien MC, Dailland T, Podeur G, Gouriou S, Schuffenecker I, Fatal case of enterovirus 71 infection, France, 2007. Emerg Infect Dis. 2009;15:1837–40. DOIPubMedGoogle Scholar
- Chumakov M, Voroshilova M, Shindarov L, Lavrova I, Gracheva L, Koroleva G, Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol. 1979;60:329–40. DOIPubMedGoogle Scholar
- Nagy G, Takatsy S, Kukan E, Mihaly I, Domok I. Virological diagnosis of enterovirus type 71 infections: experiences gained during an epidemic of acute CNS diseases in Hungary in 1978. Arch Virol. 1982;71:217–27. DOIPubMedGoogle Scholar
- Simovanian EN, Denisenko VB, Bovtalo LF, Belugina LV, Kim MA, Kukhol YS. Enterovirus infection–71 in children: clinics, diagnostics and management [in Russian]. In: Abstracts of the VI annual All-Russian Congress on Infectious Diseases; Moscow; 2014 Mar 24–26. Moscow: the Congress; 2014. p. 286–7.
- Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929–35. DOIPubMedGoogle Scholar
- Akhmadishina LV, Eremeeva TP, Trotsenko OE, Ivanova OE, Mikhailov MI, Lukashev AN. Seroepidemiology and molecular epidemiology of enterovirus 71 in Russia. PLoS ONE. 2014;9:e97404. DOIPubMedGoogle Scholar
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. DOIPubMedGoogle Scholar
- McWilliam Leitch EC, Cabrerizo M, Cardosa J, Harvala H, Ivanova OE, Koike S, The association of recombination events in the founding and emergence of subgenogroup evolutionary lineages of human enterovirus 71. J Virol. 2012;86:2676–85. DOIPubMedGoogle Scholar
- Lukashev AN, Shumilina EY, Belalov IS, Ivanova OE, Eremeeva TP, Reznik VI, Recombination strategies and evolutionary dynamics of the human enterovirus A global gene pool. J Gen Virol. 2014;95:868–73. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 21, Number 8—August 2015
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Alexander N. Lukashev, Chumakov Institute of Poliomyelitis and Viral Encephalitides, Moscow Region, Russia
Top