Volume 21, Number 8—August 2015
Synopsis
Underrecognition of Dengue during 2013 Epidemic in Luanda, Angola
Abstract
During the 2013 dengue epidemic in Luanda, Angola, 811 dengue rapid diagnostic test–positive cases were reported to the Ministry of Health. To better understand the magnitude of the epidemic and identify risk factors for dengue virus (DENV) infection, we conducted cluster surveys around households of case-patients and randomly selected households 6 weeks after the peak of the epidemic. Of 173 case cluster participants, 16 (9%) exhibited evidence of recent DENV infection. Of 247 random cluster participants, 25 (10%) had evidence of recent DENV infection. Of 13 recently infected participants who had a recent febrile illness, 7 (54%) had sought medical care, and 1 (14%) was hospitalized with symptoms consistent with severe dengue; however, none received a diagnosis of dengue. Behavior associated with protection from DENV infection included recent use of mosquito repellent or a bed net. These findings suggest that the 2013 dengue epidemic was larger than indicated by passive surveillance data.
Dengue is a potentially fatal acute febrile illness caused by any of 4 mosquito-transmitted dengue viruses (DENV-1–4). The disease is endemic throughout the tropics (1), but is underrecognized in sub-Saharan Africa (2,3), where an estimated 64 million DENV infections occurred in 2010 (4). Although dengue was identified in travelers returning from Angola in the 1980s (5), locally acquired cases had not been reported until an outbreak in 2013 that was initially thought to have resulted from importation of DENV by immigrant workers from Asia. However, the only virus detected during the outbreak was a strain of DENV-1 that molecular epidemiologic analysis indicated had been circulating in western and west-central Africa for roughly 4 decades (6–9), demonstrating regional endemicity of dengue.
During the 2013 epidemic, the Angola Ministry of Health was notified of a total of 1,214 dengue case-patients, nearly all (98%) of whom resided in the capital, Luanda, which has an estimated population of 3–14 million (Angola Ministry of Health and World Health Organization, unpub. data). Serum specimens from suspected cases were tested with a dengue rapid diagnostic test (RDT; SD BIOLINE Dengue Duo, Standard Diagnostics, Haryana, India), and positive cases were defined by detection of nonstructural protein 1 antigen, anti-DENV IgM, or both. In total, specimens from 811 (67%) persons with suspected dengue tested RDT-positive, including those from 246 (30%) hospitalized patients and from 11 (1.4%) patients who died. The highest weekly incidence occurred during May 17–23, 2013, when 125 cases were reported, of which 101 (81%) were RDT-positive.
Dengue is a focal disease, and cases frequently cluster around the households of infected persons (10,11). Previous household-based cluster investigations in Indonesia (12), Nicaragua (13), Thailand (14), and Vietnam (15) demonstrated DENV infection rates of 2.2%–12.4% among persons residing within 10–100 m of index case-patients. These studies enabled detection of unrecognized dengue cases and identification of household risk factors for DENV infection, such as the presence of uncovered water storage containers (12) and lack of piped household water supply (14). Household-based cluster investigations are therefore a useful tool to estimate the extent of dengue in regions where case reporting may be suboptimal and can also facilitate identification of local risk factors for DENV infection.
We conducted household-based cluster investigations in Luanda to detect unreported cases and identify demographic characteristics and household and behavioral risk factors for infection. Clusters consisted of households located within a 25-m radius of the following: 1) residences of dengue case-patients who sought medical care, were reported as having a suspected dengue case, and tested positive by RDT (case clusters); or 2) randomly selected households from throughout Luanda in which no known dengue case-patient resided (random clusters).
Case clusters were identified by contacting RDT-positive dengue case-patients or their parents and querying whether they were available for a household visit, which was made within 30 days of the index case-patient’s reported date of illness onset. Case clusters were studied even if the index case-patient did not participate in the investigation.
The protocol for selection of random clusters was as follows: 1) randomly selecting and traveling to 1 of 8 regions of Luanda; 2) spinning a 1-sided object (e.g., pen, bottle); 3) traveling in the indicated direction for ≈30 min by automobile without accounting for the degree of traffic congestion; 4) parking the automobile and spinning the 1-sided object again; 5) traveling by foot in the indicated direction for ≈5 min; 6) again spinning the 1-sided object; and 7) offering participation in the investigation to the nearest household in the direction indicated by the object. If the selected household was unoccupied or declined participation, the team returned to the automobile and repeated the process.
The head of the household in case and random clusters was informed of the purpose of the investigation. Households were not revisited if the head of household was unavailable. If heads of household agreed to participate in the investigation, all available household members were offered the opportunity to participate, which included the following: 1) completing a questionnaire that collected information on demographics, medical history, and mosquito avoidance strategies; and 2) providing a serum specimen for dengue diagnostic testing. Heads of household completed an additional questionnaire regarding household characteristics. All communications and questionnaires were in Portuguese. The cluster study was conducted during June 28–July 2, 2013. Specimens were processed on the day of collection, stored at −20°C, and shipped on dry ice to the Centers for Disease Control and Prevention (CDC) Dengue Branch (San Juan, Puerto Rico), for dengue diagnostic testing by real-time reverse transcription PCR (rRT-PCR) (16) and anti-DENV IgM capture ELISA (InBios International, Inc., Seattle, WA, USA). Data were compiled in a Microsoft Access (Microsoft Corp., Redmond, WA, USA) database.
Participants were household members who completed a questionnaire and provided a serum specimen. Current DENV infection was defined as detection of DENV nucleic acid by rRT-PCR. Recent DENV infection was defined as detection of anti-DENV IgM by ELISA. Dengue, dengue with warning signs, and severe dengue were defined by 2009 World Health Organization guidelines (1).
To identify differences in characteristics between recently infected participants and uninfected participants, we fitted generalized linear models with each of the variables of interest as the predictor. Random effects were included for households nested within clusters to account for correlation. Because it was unclear whether inference could be made to the greater population of Luanda, p values were computed through a permutation test, in which DENV infection statuses were permuted within households. Thus, results are only applicable to the surveyed population.
The investigation protocol underwent institutional review at CDC and was determined to be public health response and not research. As such, institutional review board approval was not required.
Serum specimens and questionnaires were collected from 455 cluster participants (Table 1). Similar numbers of households per cluster and participants per household were included in case and random clusters. Age and sex of participants had a similar distribution in case and random clusters. No participants had evidence of current DENV infection by rRT-PCR, 41 (9%) had evidence of recent DENV infection by IgM ELISA, and 35 (8%) had equivocal IgM ELISA results and were excluded from further analysis. Age and sex distributions were similar for recently-infected case and random cluster participants. Recently-infected participants from random clusters more frequently reported having fever in the past month and less frequently reported a febrile household member in the past month.
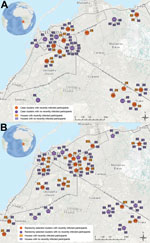
Of 173 participants from 67 households in 21 case clusters, 16 (9%) had evidence of recent DENV infection (Figure). Of 247 participants from 90 households in 26 random clusters, 25 (10%) had evidence of recent DENV infection. Most case (55%) and random (77%) clusters contained at least 1 recently infected participant. Approximately one fifth of case and random cluster households had at least 1 recently infected participant.
Recently infected participants were significantly younger and had spent significantly less time in Luanda than uninfected participants (Table 2). Roughly one third of recently infected participants and also uninfected participants reported having fever in the past 30 days. Of 13 recently infected and recently febrile participants, 5 (38%) reported symptoms consistent with dengue with warning signs (severe abdominal pain) and 1 (8%) reported symptoms consistent with severe dengue (hematemesis). Seven (54%) recently infected and recently febrile participants sought medical care; 1 (14%) was hospitalized, and none reported receiving a diagnosis of dengue. Recently infected and febrile participants who sought care frequently (71%) received a diagnosis of malaria, as were uninfected, recently febrile participants who sought care (58%).
Having used a bed net or mosquito repellent in the past 30 days were significantly associated with protection from recent DENV infection (p = 0.05 and p = 0.03, respectively; Table 2). Although most participants’ homes had piped water, delivery of household water by public water truck was also significantly associated with protection from DENV infection (p = 0.04).
In this investigation, ≈10% of case and random cluster participants had evidence of recent DENV infection. A possible explanation for why no participants had current DENV infection is that the cluster investigations were conducted ≈6 weeks after the apparent peak of the epidemic. Therefore, although DENV circulation may have been declining when surveys were conducted, anti-DENV IgM, which may persist for months after infection (17), was still detectable. This low rate of current DENV infection among cluster participants is in contrast to findings of an investigation recently conducted near the peak of a dengue outbreak in Mombasa, Kenya, in which nearly 7% of participants had evidence of current DENV infection and another 7% had recent infection (18).
Persons with evidence of recent DENV infection were most frequently 10–19 years of age, and this finding likely led to confounding in the observation that persons with recent DENV infection spent less time in Luanda than uninfected persons. Unfortunately, our sampling method did not allow for statistically valid age-matched comparisons. In addition, the expected natural history of DENV transmission in a disease-endemic area would support the idea that persons 10–19 years of age were more likely to have higher rates of infection than adults who had lived in Luanda for many years, had been previously infected with DENV-1, and were thus protected from infection in 2013. Taken together with molecular evidence of dengue endemicity in Angola (7,8), these observations indicate a level of dengue endemicity equivalent to that observed in the Americas (4), where adolescents are routinely one of the most affected age groups (19–21).
Although multiple pieces of evidence indicate that dengue is endemic in Luanda, none of the recently infected persons who sought medical care had received a diagnosis of dengue. Instead, most received a diagnosis of malaria, including 1 person who was hospitalized with an illness consistent with severe dengue. Thus, as has been observed in other regions of sub-Saharan Africa (2,18,22–24), dengue and other acute febrile illnesses may be frequently overlooked in Luanda, where malaria is in fact rare (25). Observations from this investigation therefore suggest that the number of RDT-positive dengue cases reported to the Ministry of Health was likely a large underestimation of the true magnitude of the 2013 epidemic.
An unexpected finding of this investigation was that the rate of detection of recently infected participants was equivalent in both case and random clusters. This finding is in contrast to those of most prior household cluster surveys, in which few persons or none were found to be recently infected in randomly selected clusters (13,14,26,27). One possible explanation for these differences is that the Luanda investigation was conducted in an urban environment soon after the peak of a large epidemic, whereas previous studies were conducted during periods of nonepidemic levels of transmission. Similar to results of this investigation, a recent 3-year cluster study conducted in urban Vietnam found that the incidence of having detectable anti-DENV IgM was twice as high in participants from case clusters than from control clusters (15). Alternatively, because some random clusters were surveyed on the same day and by the same teams investigating case clusters, teams may not have traveled sufficiently far from case clusters to obtain independent findings. Because dengue is a focal illness that travels in “waves” outward from urban environments (11,28), the timing and distance required to be independent from a site of known or suspected DENV transmission are unclear.
Behavior associated with protection from DENV infection included having recently used mosquito avoidance strategies (such as applying mosquito repellent or sleeping under a bed net) and delivery of household water supply by public water truck. Although the latter finding should be further investigated to both validate and explore the reasons behind its association with protection from DENV infection, use of mosquito repellent is a well-documented approach to mosquito avoidance that has been repeatedly associated with protection from DENV infection (1). Bed net use was previously associated with protection from DENV infection among soldiers in Somalia (29) but is not typically thought to be associated with protection from DENV infection because Aedes aegypti mosquitoes, which were the dominant vector detected during the Luanda epidemic (8), are most active at dusk and dawn (1). Bed net use may therefore be associated with protection from early morning biting. In line with this observation, a serosurvey in Mombasa, Kenya, conducted in 2013, found that leaving windows open at night was associated with DENV infection (18), possibly because Aedes mosquitoes enter the home in the evening and feed on the host in the early morning (30,31).
Strengths of this investigation include using community-level surveys to demonstrate the extent of dengue in a region where clinical awareness and reporting infrastructure were suboptimal. Moreover, the recent availability of an RDT enabled early detection of dengue cases, without which the epidemic may not have been recognized. Also, by using well-validated diagnostic tests to detect evidence of current or recent DENV infection in cluster participants, the likelihood that additional infections were missed is minimal. Conversely, infection with flaviviruses can result in cross-reactive antibody (32), creating the possibility of false-positive anti-DENV IgM diagnostic test results. Although the IgM ELISA used in this assay has been previously demonstrated to be highly specific for anti-DENV IgM (InBios DENV Detect IgM Capture ELISA, product insert; CDC Dengue Branch, unpub. data), it is nonetheless possible that some proportion of participants with recent DENV infection were misclassified due to false-positive diagnostic test results.
The circulation of DENV-4 was recently detected in Luanda (33,34) and may be associated with future epidemics in the region. Because early identification and proper management of dengue patients can reduce case-fatality rates among hospitalized patients from ≈10% to <0.5% (35), clinical awareness of dengue should be improved in Luanda and throughout sub-Saharan Africa through clinical dengue patient management trainings (e.g., http://www.cdc.gov/dengue/training/cme.html). Case reporting should also be improved by instituting routine laboratory-based surveillance for acute febrile illnesses in Africa, which will assist in better defining the epidemiology of dengue and other emerging infectious diseases (33,36).
Dr. Sharp is a lieutenant commander in the US Public Health Service and a health scientist at CDC Dengue Branch in San Juan, Puerto Rico. His research and public health interests include the epidemiology and pathobiology of dengue and other tropical acute febrile illnesses.
Acknowledgments
We thank Isabel Joao for assistance with field investigation logistics, Victor Luteganya for assistance with data management, Sheryl Shapiro and Jacqueline Weaver for assistance with specimen shipment, Laura Wright for assistance with map creation, and Ray A. Arthur and Serena Fuller for outbreak response support and coordination.
We acknowledge the CDC Global Disease Detection Operations Center Outbreak Response Contingency Fund for financial support of the field investigation.
References
- World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: The Organization; 2009.
- Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17:1349–54 .PubMedGoogle Scholar
- Jaenisch T, Junghanss T, Wills B, Brady OJ, Eckerle I, Farlow A, Dengue expansion in Africa-not recognized or not happening? Emerg Infect Dis. 2014;20:•••. DOIPubMedGoogle Scholar
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, The global distribution and burden of dengue. Nature. 2013;496:504–7. DOIPubMedGoogle Scholar
- Bakker RC, Veenstra J, Dingemans-Dumas AM, Wetsteyn J, Kager PA. Imported dengue in the Netherlands. J Travel Med. 1996;3:204–8. DOIPubMedGoogle Scholar
- Schwartz E, Meltzer E, Mendelson M, Tooke A, Steiner F, Gautret P, Detection on four continents of dengue fever cases related to an ongoing outbreak in Luanda, Angola, March to May 2013. Euro Surveill. 2013;18:20488 .PubMedGoogle Scholar
- Sessions OM, Khan K, Hou Y, Meltzer E, Quam M, Schwartz E, Exploring the origin and potential for spread of the 2013 dengue outbreak in Luanda, Angola. Glob Health Action. 2013;6:218–22.
- Centers for Disease Control and Prevention. Ongoing dengue epidemic—Angola, June 2013. MMWR Morb Mortal Wkly Rep. 2013;62:504–7 .PubMedGoogle Scholar
- Parreira R, Conceicao C, Centeno-Lima S, Marques N, Saraiva da Cunha J, Abreu C, Angola’s 2013 dengue outbreak: clinical, laboratory and molecular analyses of cases from four Portuguese institutions. J Infect Dev Ctries. 2014;8:1210–5. DOIPubMedGoogle Scholar
- Morrison AC, Getis A, Santiago M, Rigau-Perez JG, Reiter P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991–1992. Am J Trop Med Hyg. 1998;58:287–98 .PubMedGoogle Scholar
- Salje H, Lessler J, Endy TP, Curriero FC, Gibbons RV, Nisalak A, Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci U S A. 2012;109:9535–8. DOIPubMedGoogle Scholar
- Beckett CG, Kosasih H, Faisal I. Nurhayati, Tan R, Widjaja S, et al. Early detection of dengue infections using cluster sampling around index cases. Am J Trop Med Hyg. 2005;72:777–82.
- Reyes M, Mercado JC, Standish K, Matute JC, Ortega O, Moraga B, Index cluster study of dengue virus infection in Nicaragua. Am J Trop Med Hyg. 2010;83:683–9. DOIPubMedGoogle Scholar
- Mammen MP, Pimgate C, Koenraadt CJ, Rothman AL, Aldstadt J, Nisalak A, Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5:e205. DOIPubMedGoogle Scholar
- Anders KL, Nga LH, Thuy NT, Ngoc TV, Tam CT, Tai LT, Households as foci for dengue transmission in highly urban Vietnam. PLoS Negl Trop Dis. 2015;9:e0003528. DOIPubMedGoogle Scholar
- Santiago GA, Vergne E, Quiles Y, Cosme J, Vazquez J, Medina JF, Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis. 2013;7:e2311. DOIPubMedGoogle Scholar
- Miagostovich MP, Nogueira RM, dos Santos FB, Schatzmayr HG, Araujo ES, Vorndam V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J Clin Virol. 1999;14:183–9. DOIPubMedGoogle Scholar
- Ellis EM, Neatherlin JC, Delorey M, Ochieng M, Mohammed AH, Mogeni DO, A household aerosurvey to estimate the magnitude of a dengue outbreak in Mombasa, Kenya, 2013. PLoS Negl Trop Dis. 2015;9:e0003733 . DOIPubMedGoogle Scholar
- Sharp TM, Hunsperger E, Santiago GA, Munoz-Jordan JL, Santiago LM, Rivera A, Virus-specific differences in rates of disease during the 2010 dengue epidemic in Puerto Rico. PLoS Negl Trop Dis. 2013;7:e2159. DOIPubMedGoogle Scholar
- Villar LA, Rojas DP, Besada-Lombana S, Sarti E. Epidemiological trends of dengue disease in Colombia (2000–2011): a systematic review. PLoS Negl Trop Dis. 2015;9:e0003499. DOIPubMedGoogle Scholar
- Teixeira MG, Siqueira JB Jr, Ferreira GL, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. PLoS Negl Trop Dis. 2013;7:e2520. DOIPubMedGoogle Scholar
- Stoler J, Al Dashti R, Anto F, Fobil JN, Awandare GA. Deconstructing “malaria”: West Africa as the next front for dengue fever surveillance and control. Acta Trop. 2014;134:58–65. DOIPubMedGoogle Scholar
- Stoler J, Delimini RK, Bonney JH, Oduro AR, Owusu-Agyei S, Fobil JN, Evidence of recent dengue exposure among malaria parasite-positive children in three urban centers in Ghana. Am J Trop Med Hyg. 2015;92:497–500 . DOIPubMedGoogle Scholar
- Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7:e2324. DOIPubMedGoogle Scholar
- Thwing JI, Mihigo J, Fernandes AP, Saute F, Ferreira C, Fortes F, How much malaria occurs in urban Luanda, Angola? A health facility-based assessment. Am J Trop Med Hyg. 2009;80:487–91 .PubMedGoogle Scholar
- Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A. 2013;110:994–9. DOIPubMedGoogle Scholar
- Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJ, Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6:e1730. PMID: 22816001
- Cummings DA, Irizarry RA, Huang NE, Endy TP, Nisalak A, Ungchusak K, Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature. 2004;427:344–7. DOIPubMedGoogle Scholar
- Sharp TW, Wallace MR, Hayes CG, Sanchez JL, DeFraites RF, Arthur RR, Dengue fever in US troops during Operation Restore Hope, Somalia, 1992–1993. Am J Trop Med Hyg. 1995;53:89–94 .PubMedGoogle Scholar
- Chadee DD, Corbet PS. A night-time role of the oviposition site of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae). Ann Trop Med Parasitol. 1990;84:429–33 .PubMedGoogle Scholar
- Harrington LC, Ponlawat A, Edman JD, Scott TW, Vermeylen F. Influence of container size, location, and time of day on oviposition patterns of the dengue vector, Aedes aegypti, in Thailand. Vector Borne Zoonotic Dis. 2008;8:415–23. DOIPubMedGoogle Scholar
- Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol. 1989;70:37–43. DOIPubMedGoogle Scholar
- Parreira R, Centeno-Lima S, Lopes A, Portugal-Calisto D, Constantino A, Nina J. Dengue virus serotype 4 and chikungunya virus coinfection in a traveller returning from Luanda, Angola. Euro Surveill. 2014;19:20730 .PubMedGoogle Scholar
- Meltzer E, Lustig Y, Glichinsky O, Steiner F, Schwartz E. Probable importation of dengue virus type 4 to Angola from Brazil. Emerg Infect Dis. 2014;20:1775–6. DOIPubMedGoogle Scholar
- Lam PK, Tam DT, Diet TV, Tam CT, Tien NT, Kieu NT, Clinical characteristics of dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis. 2013;57:1577–86. DOIPubMedGoogle Scholar
- Teixeira MG, Andrade AM, Costa Mda C, Castro JN, Oliveira FL, Goes CS, East/Central/South African genotype chikungunya virus, Brazil, 2014. Emerg Infect Dis. 2015;21:906–7 PubMed. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 21, Number 8—August 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Tyler M. Sharp, Centers for Disease Control and Prevention Dengue Branch, 1324 Calle Cañada, San Juan, PR 00920-3860, USA
Top