Volume 22, Number 2—February 2016
Dispatch
The Merits of Malaria Diagnostics during an Ebola Virus Disease Outbreak
Figure 1
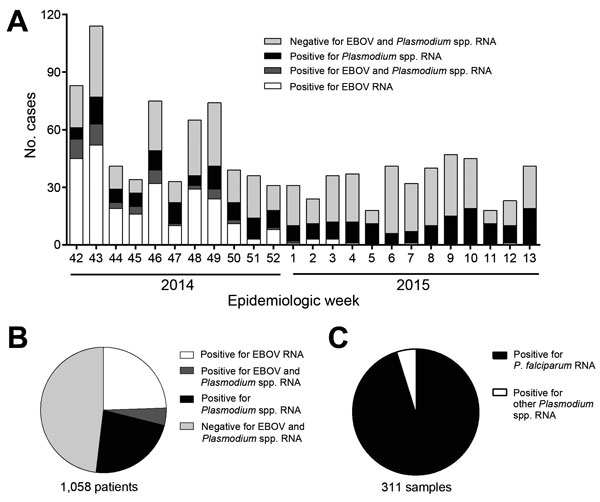
Figure 1. Prevalence of Ebola virus (EBOV) and Plasmodium spp. RNA in patient samples submitted to the Centers for Disease Control and Prevention–National Institutes of Health diagnostic laboratory at the Eternal Love Winning Africa campus in Monrovia, Liberia, from October 12, 2014 (epidemiologic week 42), through March 28, 2015 (week 13). Whole blood samples were inactivated, and RNA was extracted by using the QIAAmp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). These samples were then tested for the presence of EBOV RNA and Plasmodium spp. RNA by real-time quantitative reverse transcription PCR (qRT-PCR) (13). A) Number of patients, as determined by qRT-PCR, positive for EBOV, Plasmodium spp., both, or neither (i.e., no EBOV and no Plasmodium spp.), by epidemiologic week. B) Total number of patients receiving a laboratory diagnosis of Ebola viremia, Plasmodium spp. parasitemia, both, or neither. C) A subset of 311 Plasmodium spp. qRT-PCR–positive samples that were retested with a qRT-PCR specific for P. falciparum (14).
References
- World Health Organization. Ebola situation report. 2015 October 28 [cited 2015 Oct 28]. http://apps.who.int/iris/bitstream/10665/191299/1/ebolasitrep_28Oct2015_eng.pdf?ua=1
- Plucinski MM, Guilavogui T, Sidikiba S, Diakité N, Diakité S, Dioubaté M, Effect of the Ebola-virus-disease epidemic on malaria case management in Guinea, 2014: a cross-sectional survey of health facilities. Lancet Infect Dis. 2015;15:1017–23 .DOIPubMedGoogle Scholar
- Mobula ML, Brown CA, Burnham G, Phelps BR. Need for reinforced strategies to support delivery of HIV clinical services during the Ebola outbreak in Guinea, Liberia, and Sierra Leone. Disaster Med Public Health Prep. 2015;9:522–6 .DOIPubMedGoogle Scholar
- Takahashi S, Metcalf CJ, Ferrari MJ, Moss WJ, Truelove SA, Tatem AJ, Reduced vaccination and the risk of measles and other childhood infections post-Ebola. Science. 2015;347:1240–2 .DOIPubMedGoogle Scholar
- United Nations Population Fund. Ebola wiping out gains in safe motherhood [press release]. 16 October 2014 [cited 2015 Oct 28]. http://www.unfpa.org/news/ebola-wiping-out-gains-safe-motherhood
- Walker PG, White MT, Griffin JT, Reynolds A, Ferguson NM, Ghani AC. Malaria morbidity and mortality in Ebola-affected countries caused by decreased health-care capacity, and the potential effect of mitigation strategies: a modelling analysis. Lancet Infect Dis. 2015;15:825–32 .DOIPubMedGoogle Scholar
- World Health Organization. World malaria report 2014 [cited 2015 Oct 28]. http://www.who.int/malaria/publications/world_malaria_report_2014/report/en
- Centers for Disease Control and Prevention. Recommendations for managing and preventing cases of malaria in areas with Ebola. 2015 [cited 2015 Oct 28]. http://www.cdc.gov/vhf/ebola/outbreaks/malaria-cases.html
- Sterk E. Filovirus haemorrhagic fever guideline. Barcelona, Spain: Médecins Sans Frontières; 2008.
- Benito A, Rubio JM. Usefulness of seminested polymerase chain reaction for screening blood donors at risk for malaria in Spain. Emerg Infect Dis. 2001;7:1068 .DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. DPDx: laboratory identification of parasitic diseases of public health concern. 2015 [cited 2015 Oct 28]. http://www.cdc.gov/dpdx/diagnosticProcedures/blood/antigendetection.html
- Lee MA, Tan CH, Aw LT, Tang CS, Singh M, Lee SH, Real-time fluorescence-based PCR for detection of malaria parasites. J Clin Microbiol. 2002;40:4343–5 .DOIPubMedGoogle Scholar
- Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47:975–80 .DOIPubMedGoogle Scholar
- O’Shea MK, Clay KA, Craig DG, Matthews SW, Kao RL, Fletcher TE, Diagnosis of febrile illnesses other than Ebola virus disease at an Ebola treatment unit in Sierra Leone. Clin Infect Dis. 2015;61:795–8 .DOIPubMedGoogle Scholar
1Current affiliation: University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
2Current affiliation: Public Health Agency of Canada, Winnipeg, Manitoba, Canada.
3Current affiliation: Friedrich-Loeffler-Institut, Greifswald-Insel Riems, Germany.