Volume 22, Number 3—March 2016
Research
Decreased Time to Treatment Initiation for Multidrug-Resistant Tuberculosis Patients after Use of Xpert MTB/RIF Test, Latvia
Figure 1
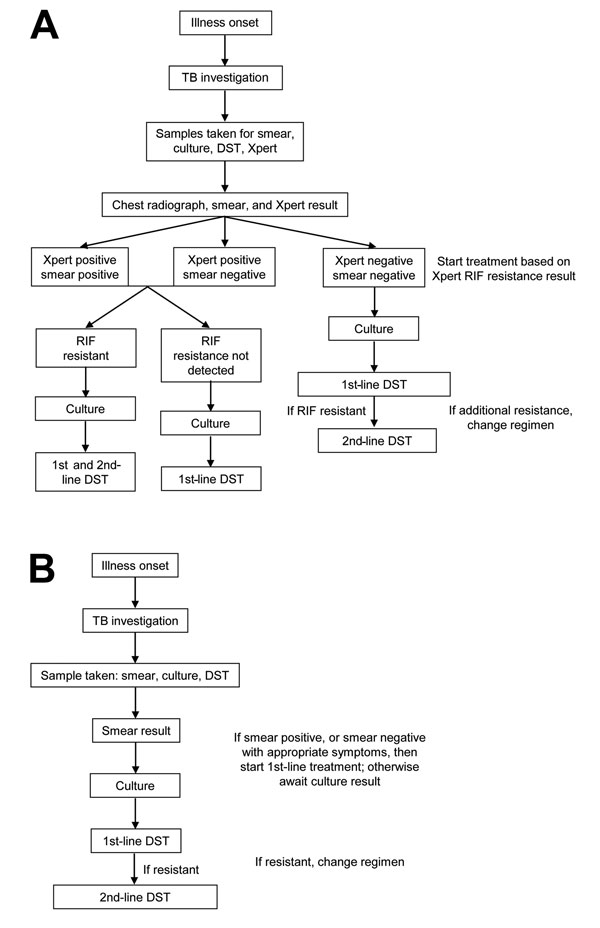
Figure 1. Diagnostic pathways for patients with multidrug-resistant tuberculosis, Latvia, 2012. A) With use of Xpert MTB/RIF; B) without use of Xpert MTB/RIF. A line probe assay was used if Xpert and DST showed discordant results. MTB, Mycobacterium tuberculosis; RIF, rifampin; TB, tuberculosis; DST, drug sensitivity testing; Xpert, Xpert MTB/RIF.
1These authors contributed equally to this article.
Page created: March 01, 2016
Page updated: March 01, 2016
Page reviewed: March 01, 2016
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.