Volume 22, Number 7—July 2016
Dispatch
Red Fox as Sentinel for Blastomyces dermatitidis, Ontario, Canada
Cite This Article
Citation for Media
Abstract
Blastomyces dermatitidis, a fungus that can cause fatal infection in humans and other mammals, is not readily recoverable from soil, its environmental reservoir. Because of the red fox’s widespread distribution, susceptibility to B. dermatitidis, close association with soil, and well-defined home ranges, this animal has potential utility as a sentinel for this fungus.
Blastomyces dermatitidis (family Ajellomycetaceae) is a fungal pathogen that causes blastomycosis, a life-threatening disease in humans, canids, and other mammals (1,2). Infection usually occurs through inhalation of conidia released from an environmental reservoir (soil) (3). In North America, high incidences of infection are reported in humans and dogs from around the Great Lakes (4). Recently, increased numbers of human blastomycosis cases have been detected in the provinces of Quebec, Saskatchewan, Manitoba, and Ontario, Canada (2,5–7). Despite this increased detection, human blastomycosis is probably underdiagnosed (2,8).
The geographic range of B. dermatitidis is based on reported clinical human cases and is therefore not clearly defined (4). B. dermatitidis is not readily recoverable or uniformly distributed within the environment, and identification has been problematic (4,9). Therefore, identifying high-risk areas for exposure has been difficult, yet this this information is crucial to minimize the number of infections.
We evaluated the utility of wild and domestic canids as potential sentinels of B. dermatitidis in the environment. We retrospectively reviewed blastomycosis case data for wildlife and companion animals in Ontario, which contains areas where blastomycosis is endemic and areas of likely emergence (2,3,5,8). Once a candidate sentinel species is identified, a targeted surveillance system can be developed to identify high-risk areas and assess risk factors associated with disease.
We analyzed blastomycosis cases diagnosed at the Animal Health Laboratory, University of Guelph (Guelph, Ontario), during 1998–2014; at 2 private diagnostic services (Guelph) during 1996–2006; and at the Canadian Wildlife Health Cooperative (Ontario regional center) during 1991–2014. Case data included date of sample collection, species, and location of carcass (wildlife) or veterinary clinic or diagnostic laboratory (companion animals). Personal privacy legislation in Canada prevented use of home addresses for companion animals. We compared animal blastomycosis data with those for 309 published human cases in Ontario during 1994–2003 (2).
Diagnoses in companion animals were made using impression smears, cytology, histopathology, serology (agar gel immunodiffusion test), and antigen detection (sandwich enzyme immunoassay). Diagnoses in wildlife were made postmortem by gross pathology and histopathology.
Blastomycosis was diagnosed in 250 companion animals (222 dogs [88.8%], 27 cats [10.8%], 1 ferret [0.4%]) and in 14 wild canids (11 red foxes [Vulpes vulpes; 78.6%], 3 gray wolves [Canis lupus; 21.4%]). Diagnoses in wild canids represent 7.4% of 149 red foxes and 1.6% of 185 wolves submitted to the Canadian Wildlife Health Cooperative (Ontario regional center) during the same period. Lungs of wild canids with blastomycosis consistently had nodules of inflammatory cells and B. dermatitidis yeasts. Less commonly, lymph nodes and skin were also affected. In red foxes found dead, B. dermatitidis was associated with severe, multifocal to coalescing, granulomatous pneumonia, whereas trapper-killed red foxes had small numbers of well-circumscribed pulmonary lesions.
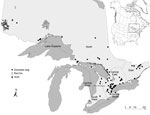
Most infected companion animals were from central regions of Ontario, as previously defined (2); however, all regions were represented (Figure). All infected wild canids were in the north region, where most human blastomycosis cases (61%; 188/309) originated during 1994–2003 (2). An additional study traced 74% (20/27) of human cases to north and east of Lake Superior (3).
Dogs were most commonly diagnosed with blastomycosis (64.4%) during July–December; the fewest (14%) were diagnosed during January–March. Most wildlife with blastomycosis (85.7%, 10/11 red foxes and 2/3 wolves) were diagnosed during November–March; the remaining 2 animals were diagnosed in September and April. Most human cases (59%, 181/309) were diagnosed during October–March (2).
B. dermatitidis infections in humans and other mammals are opportunistic and associated with contact with aerosolized conidia. Habitat sharing among humans, wildlife, and domestic animals is increasingly common (10) and provides communal opportunities for B. dermatitidis exposure. Wildlife with limited and relatively well-defined home ranges (e.g., the red fox) offer a unique opportunity to assess the distribution and, therefore, the potential risks of exposure to pathogens with an environmental reservoir.
The red fox is the most widely distributed carnivore worldwide and is common throughout much of North America, including regions with endemic as well as emerging B. dermatitidis (11). This species is highly adaptive and coexists with humans in various habitats, including recreational, residential, commercial, industrial, and urban open areas (10). Furthermore, the home range of the red fox is compact, nonoverlapping, and relatively well-defined with year-round occupancy, unlike the range of other wild canids (10,12), humans, and dogs, which can have varied and long-distance movements. The close proximity of red foxes to the ground, along with their digging, denning, and foraging behaviors, probably increases the likelihood of their close and continuous exposure to B. dermatitidis in the soil. In addition, soil in red fox dens is often sandy and acidic, a condition that, along with moisture, decaying vegetation, and animal feces, is conducive to B. dermatitidis growth (1,3,11).
Based on the co-occurrence of clinical disease in dogs and their owners, canine blastomycosis cases are a potential epidemiologic marker for the risk for human disease (9). However, in our study, pinpointing environmental exposure sources for dogs was impossible due to undisclosed travel, privacy legislation, and location variation between exposure sites and clinics. The time lag between exposure and disease onset can vary by months (13), further hindering accurate identification of exposure sources. In our study, dog blastomycosis cases mapped to Guelph are overrepresented because this southwestern Ontario city has a large veterinary hospital and diagnostic laboratory.
Although relatively few wild, compared with domestic, canids were diagnosed with blastomycosis in our study, the utility of an abundant and widespread wild canid such as the red fox as a sentinel for the risk for B. dermatitidis infection in humans should be further explored. In our study, B. dermatitidis was readily detected by gross pathology and histopathology in the lungs of red foxes, in which pulmonary lesions ranged from severe, diffuse pneumonia in foxes found dead to focal and well-circumscribed lesions in trapper-killed foxes. These findings suggest that B. dermatitidis–associated lesions in red foxes would be easily identifiable, regardless of time of year and disease manifestation. Future sampling should target foxes in northern Ontario, where risk for human infections is highest, the annual incidence is increasing, and diagnostic testing is less available (2,8,14). Furthermore, in remote northern regions, defining the range and prevalence of blastomycosis could have positive public health effects on healthcare workers and indigenous human populations.
Limitations for using wildlife for passive disease surveillance include difficulty in finding and recovering carcasses, leading to inconsistent sampling efforts. To circumvent the limitations, we suggest using carcasses of licensed fur trapper–killed red foxes for testing in targeted areas and seasons (i.e., fall to early spring). Red fox trapping is permitted year-round in southern Ontario (2) and from September to late February in the remainder of the province (15). Temporal detections of blastomycosis in humans in Ontario more closely followed detections in red foxes than in dogs.
Documenting the prevalence, distribution, seasonality, and disease manifestations of blastomycosis in red foxes in southern Ontario could help elucidate the epidemiology of this regionally emerging disease, delineate geospatial differences in exposure risks, and explore the utility of this wild canid as a sentinel for the risk to public health. Multidisciplinary research such as this provides opportunities for the development of partnerships among public health and medical researchers, physicians, veterinarians, biologists, epidemiologists, natural resource managers, and hunter and trapper federations with the common goal of reducing disease risks.
Dr. Nemeth is an assistant professor in the Department of Pathobiology at the University of Guelph. Her research interests include vectorborne disease ecology and wildlife and zoonotic diseases.
Acknowledgment
We thank the veterinarians, clinicians, pathologists, biologists, and public for the submission and interpretation of blastomycosis case material, as well as the Canadian Wildlife Health Cooperative colleagues for logistical support. Some case data were contributed by Histovet, Guelph, Ontario, Canada. Colleagues at the Ministry of Natural Resources and Forestry, Kenora District; Parks Canada; Pukaskwa National Park; and Ministry of Natural Resources and Forestry, Parry Sound, Ontario, Canada, coordinated early wildlife submissions.
References
- Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract. 2003;33:721–7. DOIPubMedGoogle Scholar
- Morris SK, Brophy J, Richardson SE, Summerbell R, Parkin PC, Jamieson F, Blastomycosis in Ontario, 1994–2003. Emerg Infect Dis. 2006;12:274–9. DOIPubMedGoogle Scholar
- Kane J, Righter J, Krajden S, Lester RS. Blastomycosis: a new endemic focus in Canada. Can Med Assoc J. 1983;129:728–31.PubMedGoogle Scholar
- Reed KD, Meece JK, Archer JR, Peterson AT. Ecologic niche modeling of Blastomyces dermatitidis in Wisconsin. PLoS One. 2008;3:e2034. DOIPubMedGoogle Scholar
- Lester RS, DeKoven JG, Kane J, Simor AE, Krajden S, Summerbell RC. Novel cases of blastomycosis acquired in Toronto, Ontario. CMAJ. 2000;163:1309–12.PubMedGoogle Scholar
- Litvinov IV, St-Germain G, Pelletier R, Paradis M, Sheppard DC. Endemic human blastomycosis in Quebec, Canada, 1988–2011. Epidemiol Infect. 2013;141:1143–7. DOIPubMedGoogle Scholar
- Davies JL, Epp T, Burgess HJ. Prevalence and geographic distribution of canine and feline blastomycosis in the Canadian prairies. Can Vet J. 2013;54:753–60.PubMedGoogle Scholar
- Dalcin D, Ahmed Z. Blastomycosis in northwestern Ontario, 2004 to 2014. Can J Infect Dis Med Microbiol. 2015;26:259–62 .DOIPubMedGoogle Scholar
- MacDonald PDM, Langley RL, Gerkin SR, Torok MR, MacCormack JN. Human and canine pulmonary blastomycosis, North Carolina, 2001–2002. Emerg Infect Dis. 2006;12:1242–4. DOIPubMedGoogle Scholar
- Rosatte R, Allan M. The ecology of red foxes, Vulpes vulpes, in metropolitan Toronto, Ontario: disease management implications. Can Field Nat. 2009;123:215–20.
- Trewhella WJ, Harris S, McAllister FE. Dispersal distance, home range size and population density in the red fox (Vulpes vulpes): a quantitative analysis. J Appl Ecol. 1988;25:423–34. DOIGoogle Scholar
- DiSalvo AF. Blastomycosis. In: Al-Doory Y, DiSalvo AF, editors. The ecology of Blastomyces dermatitidis. New York: Plenum Medical Book Co.; 1992. p. 42–73.
- McIntyre L. Blastomycosis in northwestern Ontario. Can Fam Physician. 1985;31:161–4.PubMedGoogle Scholar
- Ontario Ministry of Natural Resources. Hunting regulations summary. 2013 [cited 2015 Mar 31]. https://www.ontario.ca/environment-and-energy/2013-furbearing-mammals-seasons-hr-summary
Figure
Cite This ArticleTable of Contents – Volume 22, Number 7—July 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nicole M. Nemeth, Department of Pathobiology, University of Guelph, 419 Gordon St, Guelph, ON N1G2W1, Canada
Top