Volume 23, Number 12—December 2017
Research Letter
New Avian Hepadnavirus in Palaeognathous Bird, Germany
Figure
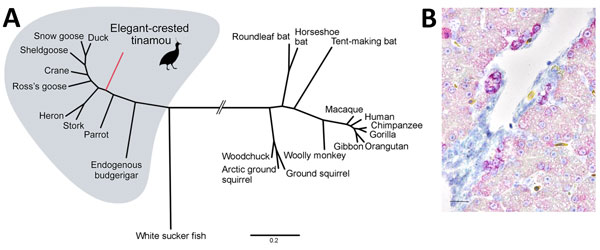
Figure. Phylogenetic and histopathologic analysis of probable new avian hepadnavirus, elegant-crested tinamou hepatitis B virus (ETHBV), Germany. A) Bayesian phylogeny of virus isolated from an elegant-crested tinamou (Eudromia elegans) compared with reference viruses. Tree was created on the basis of full-genome sequences from the family Hepadnaviridae. The analysis was run for 4 million generations and sampled every 100 steps, and the first 25% of samples were discarded as burn-in in MrBayes (7). Hasegawa-Kishino-Yano nucleotide substitution model was selected as best-fit model according to Bayesian information criteria. Posterior probabilities are shown in Technical Appendixhttps://wwwnc.cdc.gov/EID/article/23/12/16-1634-F1.htm). B) ETHBV-specific RNA (in red; Fast Red) localized within hepatocytes of the liver tissue of an elegant-crested tinamou embryo by in situ hybridization (Technical Appendix). Positive signal is enhanced in hepatocytes localized close to the vessels and negative in endothelial cells. Nonprobe incubation of the tinamou and liver tissue from a pheasant were used as negative controls. Scale bar indicates 40 μm.
References
- Funk A, Mhamdi M, Will H, Sirma H. Avian hepatitis B viruses: molecular and cellular biology, phylogenesis, and host tropism. World J Gastroenterol. 2007;13:91–103. DOIPubMedGoogle Scholar
- Hahn CM, Iwanowicz LR, Cornman RS, Conway CM, Winton JR, Blazer VS. Characterization of a novel hepadnavirus in the white sucker (Catostomus commersonii) from the Great Lakes region of the United States. J Virol. 2015;89:11801–11. DOIPubMedGoogle Scholar
- Liu W, Pan S, Yang H, Bai W, Shen Z, Liu J, et al. The first full-length endogenous hepadnaviruses: identification and analysis. J Virol. 2012;86:9510–3. DOIPubMedGoogle Scholar
- Suh A, Brosius J, Schmitz J, Kriegs JO. The genome of a Mesozoic paleovirus reveals the evolution of hepatitis B viruses. Nat Commun. 2013;4:1791. DOIPubMedGoogle Scholar
- Suh A, Weber CC, Kehlmaier C, Braun EL, Green RE, Fritz U, et al. Early mesozoic coexistence of amniotes and hepadnaviridae. PLoS Genet. 2014;10:e1004559. DOIPubMedGoogle Scholar
- Chang SF, Netter HJ, Hildt E, Schuster R, Schaefer S, Hsu YC, et al. Duck hepatitis B virus expresses a regulatory HBx-like protein from a hidden open reading frame. J Virol. 2001;75:161–70. DOIPubMedGoogle Scholar
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–5. DOIPubMedGoogle Scholar
- Pfankuche VM, Bodewes R, Hahn K, Puff C, Beineke A, Habierski A, et al. Porcine bocavirus infection associated with encephalomyelitis in a pig, Germany. Emerg Infect Dis. 2016;22:1310–2. DOIPubMedGoogle Scholar
- Prassolov A, Hohenberg H, Kalinina T, Schneider C, Cova L, Krone O, et al. New hepatitis B virus of cranes that has an unexpected broad host range. J Virol. 2003;77:1964–76. DOIPubMedGoogle Scholar
1These authors contributed equally to this article.