Volume 23, Number 9—September 2017
Research
Molecular Antimicrobial Resistance Surveillance for Neisseria gonorrhoeae, Northern Territory, Australia
Figure 2
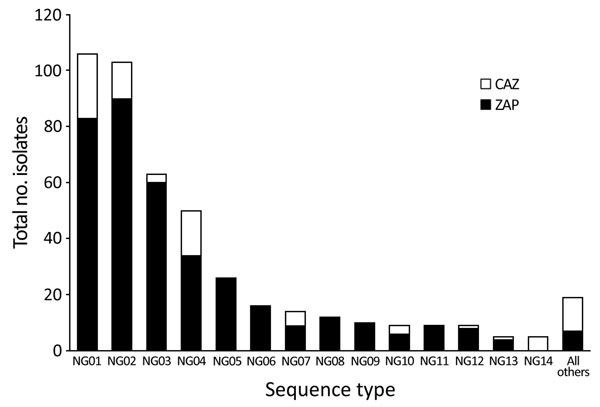
Figure 2. Genotype frequency of the 456 Neisseria gonorrhoeae clinical samples taken from patients in the Northern Territory of Australia, 2014, that were successfully genotyped by using the iPLEX14SNP method (9). Presence of each genotype in the CAZ or ZAP regions is indicated. CAZ, ceftriaxone via intramuscular injection and oral azithromycin; ZAP, azithromycin, amoxicillin, probenecid.
References
- World Health Organization. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae, 2012 [cited 2016 May 25]. http://www.who.int/reproductivehealth/publications/rtis/9789241503501/en/
- Centers for Disease Control and Prevention. Cephalosporin-resistant Neisseria gonorrhoeae public health response plan 2012 [cited 2016 Nov 11]. https://www.cdc.gov/std/treatment/ceph-r-responseplanjuly30-2012.pdf
- Goire N, Lahra MM, Chen M, Donovan B, Fairley CK, Guy R, et al. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol. 2014;12:223–9. DOIPubMedGoogle Scholar
- Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, Watanabe H, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148–9. DOIPubMedGoogle Scholar
- Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56:1273–80. DOIPubMedGoogle Scholar
- Lahra MM, Ryder N, Whiley DM. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med. 2014;371:1850–1. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590–4.PubMedGoogle Scholar
- Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, et al. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med. 2016;374:2504–6. DOIPubMedGoogle Scholar
- Trembizki E, Smith H, Lahra MM, Chen M, Donovan B, Fairley CK, et al. High-throughput informative single nucleotide polymorphism-based typing of Neisseria gonorrhoeae using the Sequenom MassARRAY iPLEX platform. J Antimicrob Chemother. 2014;69:1526–32. DOIPubMedGoogle Scholar
- Lahra MM; Australian Gonococcal Surveillance Programme. Australian Gonococcal Surveillance Programme annual report, 2014. Commun Dis Intell Q Rep. 2015;39:E347–54.PubMedGoogle Scholar
- Buckley C, Trembizki E, Baird RW, Chen M, Donovan B, Freeman K, et al. Multitarget PCR assay for direct detection of penicillinase-producing Neisseria gonorrhoeae for enhanced surveillance of gonococcal antimicrobial resistance. J Clin Microbiol. 2015;53:2706–8. DOIPubMedGoogle Scholar
- Goire N, Freeman K, Tapsall JW, Lambert SB, Nissen MD, Sloots TP, et al. Enhancing gonococcal antimicrobial resistance surveillance: a real-time PCR assay for detection of penicillinase-producing Neisseria gonorrhoeae by use of noncultured clinical samples. J Clin Microbiol. 2011;49:513–8. DOIPubMedGoogle Scholar
- Speers DJ, Fisk RE, Goire N, Mak DB. Non-culture Neisseria gonorrhoeae molecular penicillinase production surveillance demonstrates the long-term success of empirical dual therapy and informs gonorrhoea management guidelines in a highly endemic setting. J Antimicrob Chemother. 2014;69:1243–7. DOIPubMedGoogle Scholar
- Buckley C, Trembizki E, Donovan B, Chen M, Freeman K, Guy R, et al.; GRAND Study Investigators. A real-time PCR assay for direct characterization of the Neisseria gonorrhoeae GyrA 91 locus associated with ciprofloxacin susceptibility. J Antimicrob Chemother. 2016;71:353–6. DOIPubMedGoogle Scholar
- Trembizki E, Guy R, Donovan B, Kaldor JM, Lahra MM, Whiley DM; GRAND study investigators. Further evidence to support the individualised treatment of gonorrhoea with ciprofloxacin. Lancet Infect Dis. 2016;16:1005–6. DOIPubMedGoogle Scholar
- Trembizki E, Buckley C, Donovan B, Chen M, Guy R, Kaldor J, et al. Direct real-time PCR-based detection of Neisseria gonorrhoeae 23S rRNA mutations associated with azithromycin resistance. J Antimicrob Chemother. 2015;70:3244–9.PubMedGoogle Scholar
- Buckley C, Trembizki E, Donovan B, Chen M, Freeman K, Guy R, et al.; Gonorrhoea Resistance Assessment by Nucleic Acid Detection (GRAND) Study Investigators. Real-time PCR detection of Neisseria gonorrhoeae susceptibility to penicillin. J Antimicrob Chemother. 2016;71:3090–5. DOIPubMedGoogle Scholar
- Kugelman G, Tapsall JW, Goire N, Syrmis MW, Limnios A, Lambert SB, et al. Simple, rapid, and inexpensive detection of Neisseria gonorrhoeae resistance mechanisms using heat-denatured isolates and SYBR green-based real-time PCR. Antimicrob Agents Chemother. 2009;53:4211–6. DOIPubMedGoogle Scholar
- Palmer HM, Young H, Graham C, Dave J. Prediction of antibiotic resistance using Neisseria gonorrhoeae multi-antigen sequence typing. Sex Transm Infect. 2008;84:280–4. DOIPubMedGoogle Scholar
- Whiley DM, Goire N, Ray ES, Limnios A, Lambert SB, Nissen MD, et al. Neisseria gonorrhoeae multi-antigen sequence typing using non-cultured clinical specimens. Sex Transm Infect. 2010;86:51–5. DOIPubMedGoogle Scholar
- Chisholm SA, Unemo M, Quaye N, Johansson E, Cole MJ, Ison CA, et al. Molecular epidemiological typing within the European Gonococcal Antimicrobial Resistance Surveillance Programme reveals predominance of a multidrug-resistant clone. Euro Surveill. 2013;18:18.PubMedGoogle Scholar
- Guy R, Ward J, Wand H, Rumbold A, Garton L, Hengel B, et al.; STRIVE Investigator Group. Coinfection with Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis: a cross-sectional analysis of positivity and risk factors in remote Australian Aboriginal communities. Sex Transm Infect. 2015;91:201–6. DOIPubMedGoogle Scholar
- Trembizki E, Wand H, Donovan B, Chen M, Fairley CK, Freeman K, et al. The molecular epidemiology and antimicrobial resistance of Neisseria gonorrhoeae in Australia: a nationwide cross-sectional study, 2012. Clin Infect Dis. 2016;63:1591–8. DOIPubMedGoogle Scholar
Page created: August 14, 2017
Page updated: August 14, 2017
Page reviewed: August 14, 2017
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.