Volume 24, Number 6—June 2018
Research
Prion Disease in Dromedary Camels, Algeria
Figure 1
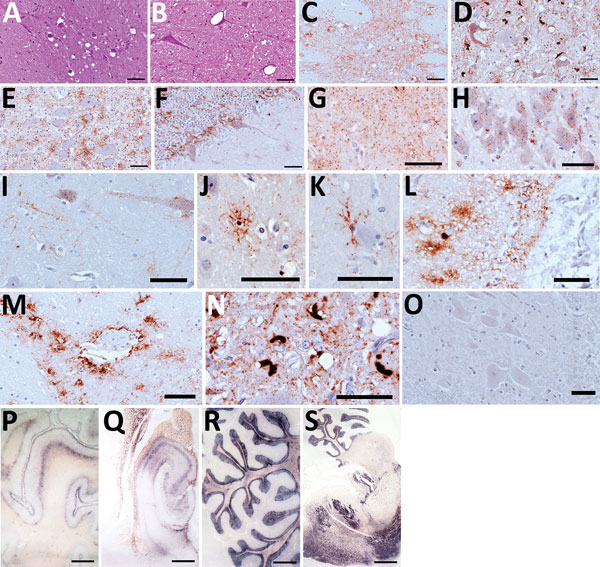
Figure 1. Hematoxylin and eosin staining (A, B), immunohistochemistry (C–O), and paraffin-embedded tissue blot analysis (P–S) of brains of dromedary camels brought for slaughter to the Ouargla abattoir, Algeria, 2016–2017. Spongiform change of neuropil, gliosis, and neuronal loss in thalamus (A) and intraneuronal vacuolation in pons (B) (scale bar = 50 μm). Immunohistochemistry for prion protein (PrPSc) with L42 monoclonal antibody evidenced dense synaptic/punctate deposition in thalamus (C) and intraneuronal and extraneuronal PrPSc deposits in pons (D), accompanied by spongiform change. Perineuronal, diffused in neuropil and glial-associated PrPSc staining were also observed in the nucleus of the solitary tract (E) and cerebellum (F), which showed rare vacuoles (scale bars = 50 μm). Immunohistochemical analysis performed on brains of symptomatic dromedaries revealed several PrPSc deposition patterns, such as synaptic/punctate pattern diffused in the neuropil (G); intraneuronal deposition in pyramidal cells of hippocampus (H); perineuronal and linear staining in frontal cortex (I); intraglial PrPSc deposition (J–L); perivascular deposition (M); atypical intracellular PrPSc deposition pattern in pons (N). PrPSc was absent in asymptomatic dromedary used as negative control (O) (scale bars = 50 μm). PrPSc distribution, by paraffin-embedded tissue blot analysis, was observed in several brain areas, such as prefrontal cortex (P), hippocampus (Q), cerebellum (R), and a sagittal section of pons (S) (scale bar = 3 mm).