Volume 24, Number 6—June 2018
Research
Influenza D Virus Infection in Feral Swine Populations, United States
Figure 3
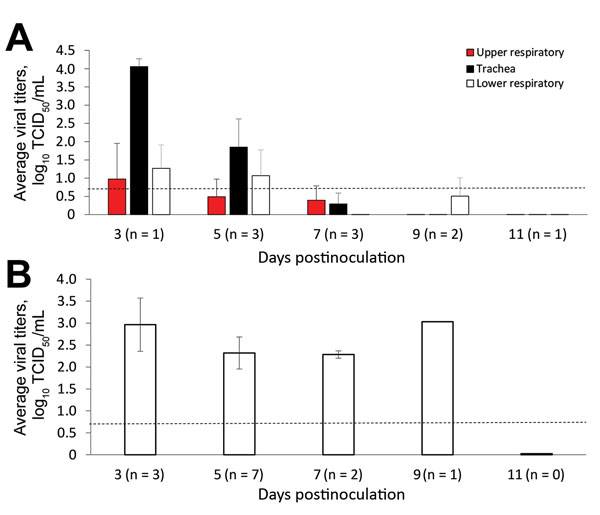
Figure 3. Influenza D viral titers in feral swine tissues. Feral swine were inoculated intranasally with 106 TCID50 of influenza D/bovine/C00046N/Mississippi/2014 virus or sterile phosphate-buffered saline (controls). At 3, 5, 7, 9, and 11 days postinoculation, they were humanely euthanized, and the following tissues were collected: turbinate; soft palate; upper, middle, and lower trachea; bronchus; left and right caudal lung, left and right medial lung, left and right cranial lung; and right accessory lung. A) The tissues were grouped as upper respiratory tract (turbinate and soft palate), trachea (upper, middle, and lower trachea, and bronchus), and lower respiratory tract (left and right caudal lung; left and right medial lung; and left and right cranial lung). B) All lung tissue sections at each time point that were influenza D virus–positive by TCID50 titration in HRT-18G cells were averaged and plotted for each day postinoculation; day 9 has no error bars because only 1 positive tissue sample was found. Dashed lines indicate the lower limit of detection, which was 100.699 TCID50/mL. Error bars indicate SE. Numbers in parentheses indicate number of animals used in the analyses. TCID50, 50% tissue culture infective dose.
1These authors contributed equally to this article.
2Current affiliation: South China Agricultural University, Guangzhou, China.