Volume 24, Number 8—August 2018
Dispatch
Fatal Nongroupable Neisseria meningitidis Disease in Vaccinated Patient Receiving Eculizumab
Figure 1
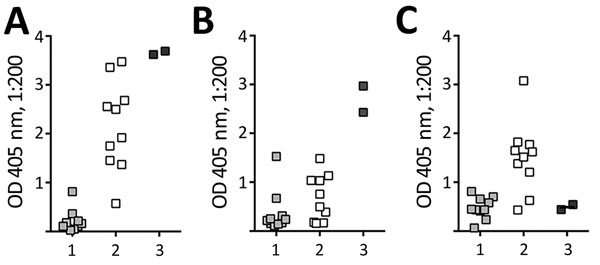
Figure 1. Serum IgG reactivity to 3 recombinant antigens in Neisseria meningitidis serogroup B meningococcal vaccine (MenB-4C) (Bexsero; GlaxoSmithKline, Bellaria Rosia, Sovicille, Italy), determined by ELISA. A postmortem serum sample (3) from a 16-year-old girl with paroxysmal nocturnal hemoglobinuria who died of meningococcal disease after treatment with eculizumab. Reactivity was measured in parallel with stored serum from 10 unvaccinated college students (1) and 10 vaccinated college students (2) 7 months after vaccination with MenB-4C (8). For the comparison specimens, each data point represents reactivity of an individual person. A) Factor H binding protein. B) Neisseria heparin binding antigen. C) NadA, MenB-4C antigens absent. OD, optical density. Data points for (3) indicate results of replicate assays. OD, optical density.
References
- Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, et al.; HUS International. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39. DOIPubMedGoogle Scholar
- Alexion Pharmaceuticals, Inc. Soliris. Revised 1/2016 [package insert] [cited 2016 Dec 2]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125166s417lbl.pdf
- Alexion Pharmaceuticals, Inc. Soliris REMS (Risk Evaluation and Mitigation Strategy) [cited 2017 Jul 23]. http://www.solirisrems.com
- Lebel E, Trahtemberg U, Block C, Zelig O, Elinav H. Post-eculizumab meningococcaemia in vaccinated patients. Clin Microbiol Infect. 2018;24:89–90. DOIPubMedGoogle Scholar
- Cullinan N, Gorman KM, Riordan M, Waldron M, Goodship TH, Awan A. Case report: Benefits and challenges of long-term eculizumab in atypical hemolytic uremic syndrome. Pediatrics. 2015;135:e1506–9. DOIPubMedGoogle Scholar
- Struijk GH, Bouts AH, Rijkers GT, Kuin EA, ten Berge IJ, Bemelman FJ. Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am J Transplant. 2013;13:819–20. DOIPubMedGoogle Scholar
- Partridge E, Lujan E, Giuntini S, Vu DM, Granoff DM. The role of anti-NHba antibody in bactericidal activity elicited by the meningococcal serogroup B vaccine, MenB-4C. Vaccine. 2017;35:4236–44. DOIPubMedGoogle Scholar
- Lujan E, Winter K, Rovaris J, Liu Q, Granoff DM. Serum bactericidal antibody responses of students immunized with a meningococcal serogroup B vaccine in response to an outbreak on a university campus. Clin Infect Dis. 2017;65:1112–9. DOIPubMedGoogle Scholar
- Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol. 2011;186:3606–14. DOIPubMedGoogle Scholar
- Hillmen P, Hall C, Marsh JC, Elebute M, Bombara MP, Petro BE, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350:552–9. DOIPubMedGoogle Scholar
- Konar M, Granoff DM. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood. 2017;130:891–9. DOIPubMedGoogle Scholar
- Sprong T, Brandtzaeg P, Fung M, Pharo AM, Høiby EA, Michaelsen TE, et al. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood. 2003;102:3702–10. DOIPubMedGoogle Scholar
- McNamara LATN, Topaz N, Wang X, Hariri S, Fox L, MacNeil JR. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734–7. DOIPubMedGoogle Scholar
- Yazdankhah SP, Kriz P, Tzanakaki G, Kremastinou J, Kalmusova J, Musilek M, et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J Clin Microbiol. 2004;42:5146–53. DOIPubMedGoogle Scholar
- Marsh JW, Shutt KA, Pajon R, Tulenko MM, Liu S, Hollick RA, et al. Diversity of factor H-binding protein in Neisseria meningitidis carriage isolates. Vaccine. 2011;29:6049–58. DOIPubMedGoogle Scholar