Treatment Outcomes in Global Systematic Review and Patient Meta-Analysis of Children with Extensively Drug-Resistant Tuberculosis
Muhammad Osman

, Elizabeth P. Harausz, Anthony J. Garcia-Prats, H. Simon Schaaf, Brittany K. Moore, Robert M. Hicks
1, Jay Achar, Farhana Amanullah, Pennan Barry, Mercedes Becerra, Domnica I. Chiotan, Peter C. Drobac
2, Jennifer Flood, Jennifer Furin, Medea Gegia, Petros Isaakidis, Andrei Mariandyshev, Iveta Ozere, N. Sarita Shah
3, Alena Skrahina, Elena Yablokova, James A. Seddon
4, Anneke C. Hesseling
4, and for The Collaborative Group for Meta-Analysis of Paediatric Individual Patient Data in MDR TB
Author affiliations: Stellenbosch University, Cape Town, South Africa (M. Osman, A.J. Garcia-Prats, H.S. Schaaf, J.A. Seddon, A.C. Hesseling); State University of New York Upstate Medical University, Syracuse, New York, USA (E.P. Harausz); Centers for Disease Control and Prevention, Atlanta, Georgia, USA (B.K. Moore); University of California, San Francisco, California, USA (R.M. Hicks); Médecins Sans Frontières, London, UK (J. Achar); Indus Hospital, Karachi, Pakistan (F. Amanullah); California Department of Public Health, Richmond, California, USA (P. Barry, J. Flood); Harvard Medical School, Boston, Massachusetts, USA (M. Becerra, P.C. Drobac, J. Furin); Romanian National TB Program, Bucharest, Romania (D.I. Chiotan); World Health Organization, Geneva, Switzerland (M. Gegia); Médecins Sans Frontières, Mumbai, India (P. Isaakidis); Northern State Medical University, Arkhangelsk, Russia (A. Mariandyshev, E. Yablokova); Riga Eastern Clinical University Hospital, Riga, Latvia (I. Ozere); Albert Einstein College of Medicine, Bronx, New York, USA (N.S. Shah); Republican Research and Practical Centre for Pulmonology and TB, Minsk, Belarus (A. Skrahina); Imperial College London, London (J.A. Seddon)
Main Article
Figure 3
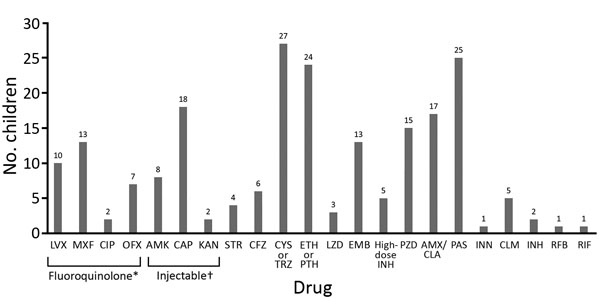
Figure 3. Drugs used for treatment of children with extensively drug resistant tuberculosis. *Includes moxifloxacin, levofloxacin, ofloxacin or ciprofloxacin. †Includes second-line injectable drugs kanamycin, amikacin, or capreomycin. AMK, amikacin, AMX, amoxicillin; CAP, capreomycin; CFZ, clofazimine; CIP, ciprofloxacin; CLA, clavulanic acid; CLM, clarithromycin; CYS, cycloserine; EMB, ethambutol; ETH, ethionamide; INH, isoniazid; INN, thioacetazone; KAN, kanamycin; LVX, levofloxacin; LZD, linezolid; MXF, moxifloxacin; OFX, ofloxacin; PAS, para-aminosalicylic acid; PTH, prothionamide; PZA, pyrazinamide; RFB, fifabutin; RIF, rifampin; STR, streptomycin; TRZ, terizidone.
Main Article
Page created: February 19, 2019
Page updated: February 19, 2019
Page reviewed: February 19, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
